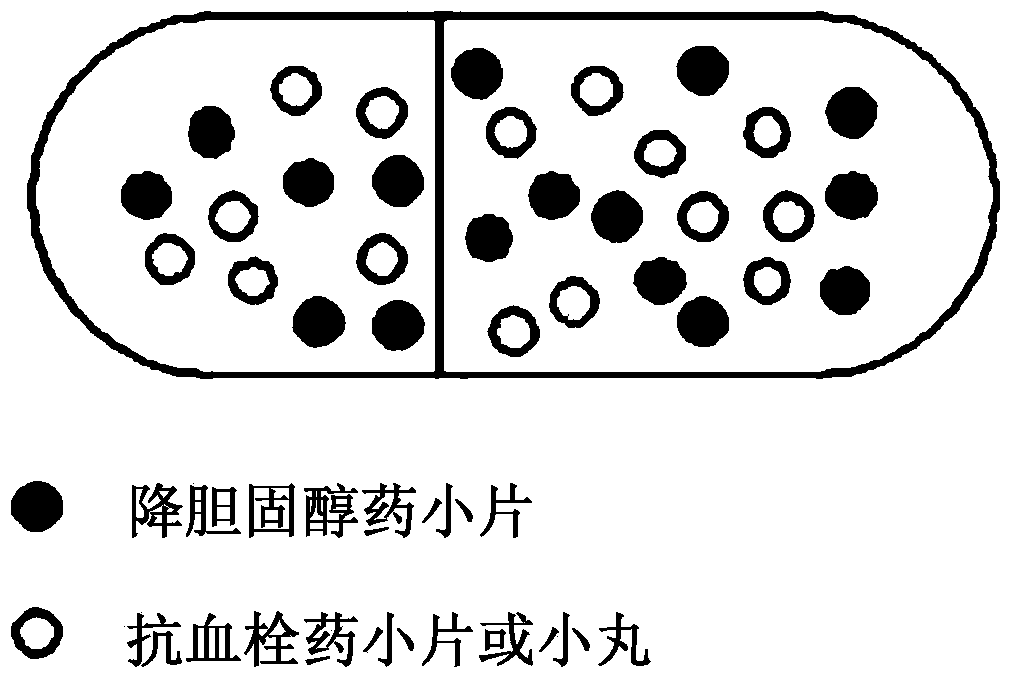
Combined formulation with improved stability
A stabilizer and preparation technology, which is applied in the field of oral administration combination preparations, can solve problems such as the difficulty in preparing the middle layer and the inability to ensure the uniformity of the middle layer, and achieve the effects of inhibiting platelet aggregation, improving storage stability, and preventing the formation of thrombus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example A-1
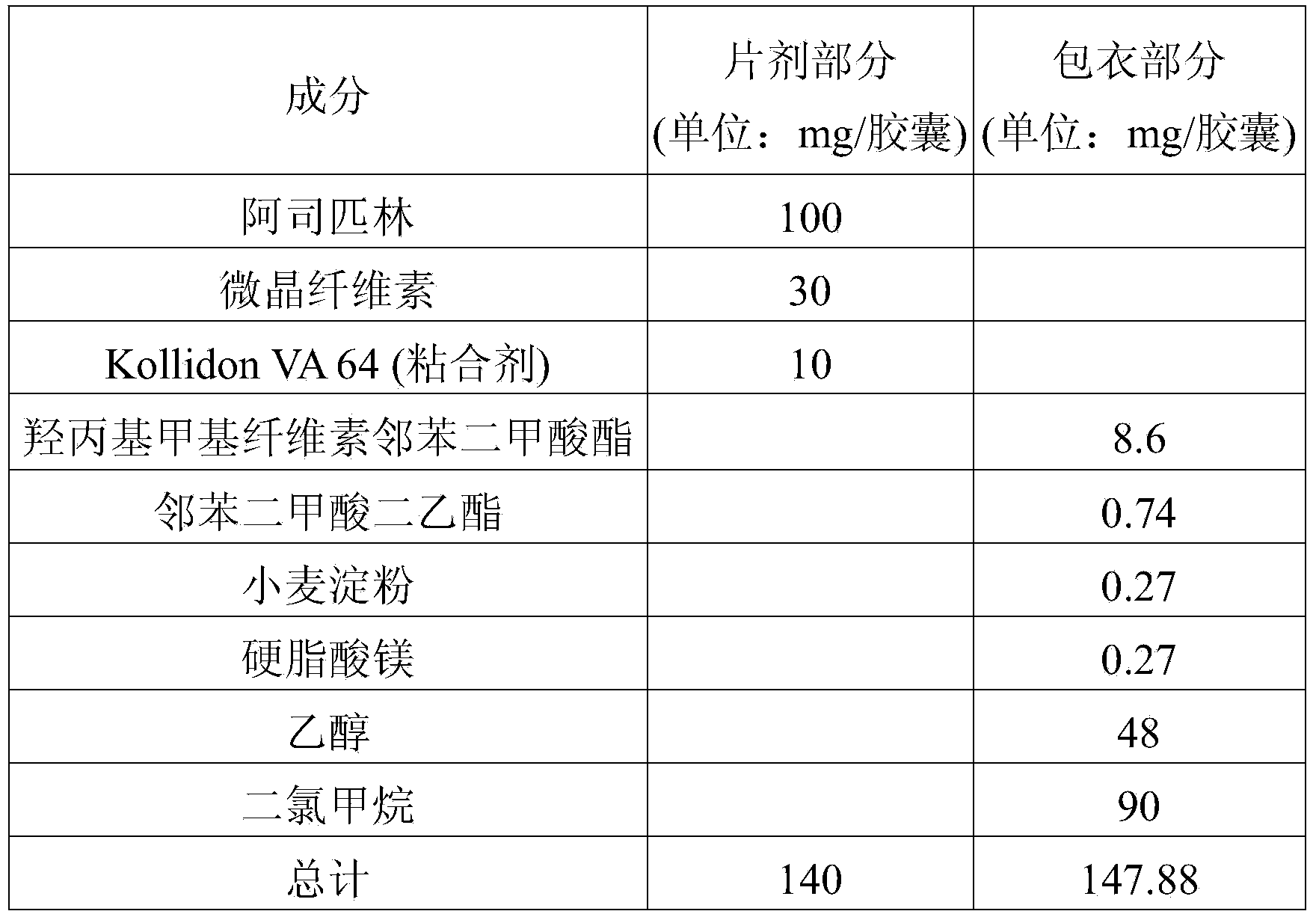
[0092] Preparation example A-1 (preparation of small aspirin tablets)
[0093] The ingredients and amounts of the aspirin minitablets used in the pharmaceutical dosage form of the present invention are shown in Table 1 below.
[0094] [Table 1]
[0095] Composition of aspirin tablets
[0096]
[0097] (1) Preparation of small pieces
[0098] Aspirin, microcrystalline cellulose and Kollidon VA64 (obtained from BASF) were mixed in the amounts shown in Table 1 using a V-shaped mixer, and the resulting mixture was then placed in a tablet press equipped with a multi-head punch (KT-10S, available in from Sejong Pharmatech) and compressed under a pressure of 1 KN to prepare round pellets with a diameter of 1.5-7.5 mm and a weight of 2-50 mg.
[0099] (2) Enteric coating
[0100] Mix the pharmaceutically acceptable enteric coating material (hydroxypropyl methylcellulose phthalate, diethyl phthalate, wheat starch and magnesium stearate) in the amount shown in Table 1, Dissolve ...
preparation example A-2
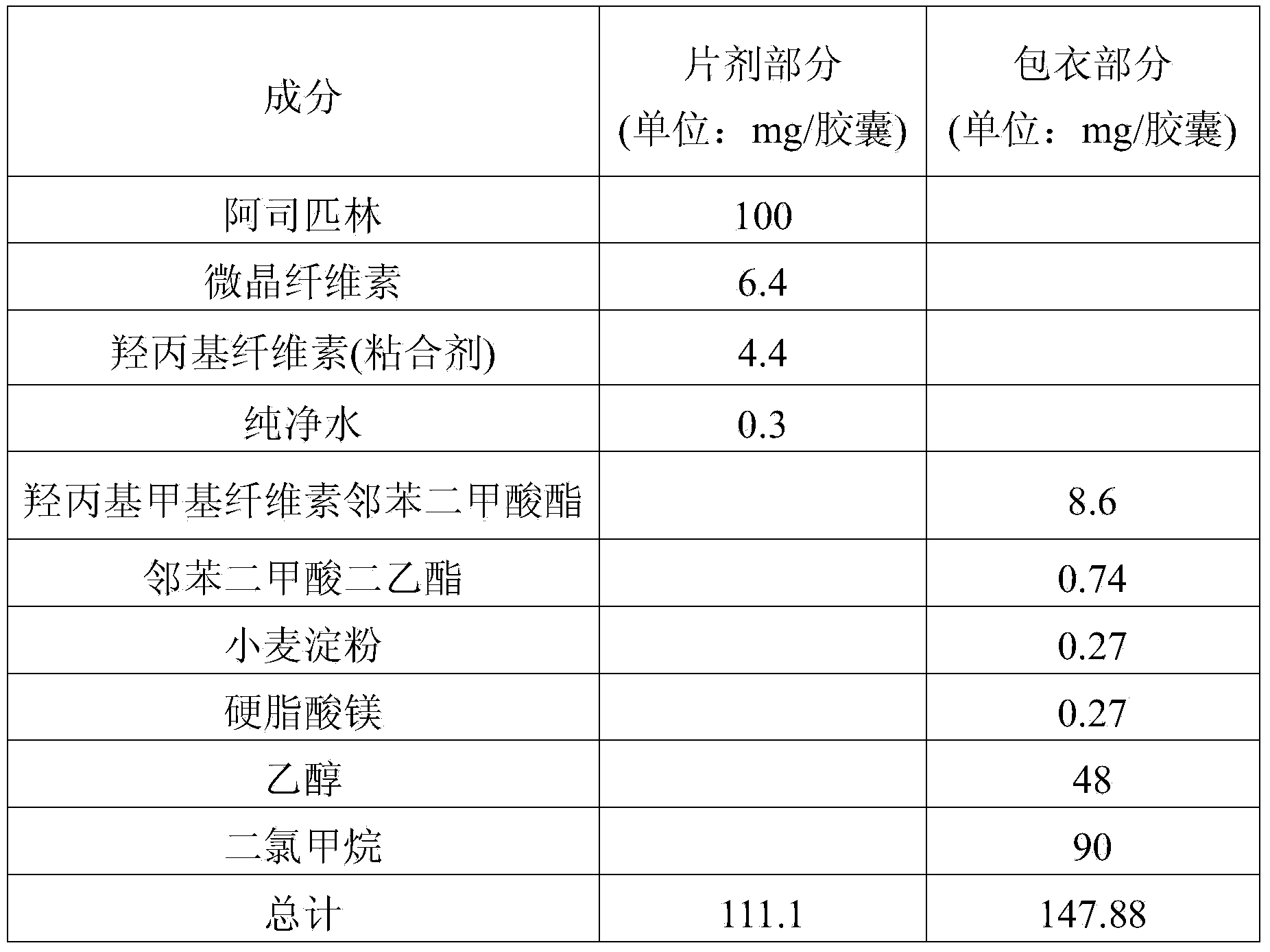
[0101] Preparation example A-2 (preparation of small aspirin tablets)
[0102] The ingredients and amounts of the aspirin minitablets used in the pharmaceutical dosage form of the present invention are shown in Table 2 below.
[0103] [Table 2]
[0104] Composition of aspirin tablets
[0105]
[0106] (1) Preparation of small pieces
[0107] Aspirin and microcrystalline cellulose were mixed in the amounts shown in Table 2 using a high-speed mixer (available from Sejong Pharmatech), and then granulated with the previously prepared binder solution (hydroxypropylcellulose solution dispersed in purified water). The granules thus obtained were dried, sieved to have a predetermined particle size (mesh opening: 25-30 mesh), and mixed using a V-shaped mixer, and then the mixture was placed in a tablet press equipped with a multi-head punch (KT-10S , from Sejong Pharmatech) and compressed under a pressure of 1 KN to prepare round pellets with a diameter of 1.5-7.5 mm and a weight...
preparation example A-3
[0110] Preparation A-3 (preparation of aspirin pellets)
[0111] The ingredients and amounts of the aspirin pellets used in the pharmaceutical dosage form of the present invention are shown in Table 3 below.
[0112] [table 3]
[0113] Composition of aspirin pellets
[0114]
[0115] (1) Preparation of aspirin pellets
[0116] Aspirin and microcrystalline cellulose were mixed in the amounts of Table 3 using a high-speed mixer (available from Sejong Pharmatech), and granulated with the previously prepared binder solution (hydroxypropylcellulose solution dispersed in purified water). The granules thus obtained were extruded with an extruder (from Sejong Pharmatech) and spheronized using a spherical granulator (Marumerizer, from Sejong Pharmatech) to prepare spherical pellets.
[0117] (2) Enteric coating
[0118] The pharmaceutically acceptable enteric coating material (hydroxypropyl methylcellulose phthalate, diethyl phthalate, wheat starch and magnesium stearate) was m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com