Memantine hydrochloride sustained release preparation and preparing method thereof
A technology for memantine hydrochloride and sustained-release preparations, which is applied in pharmaceutical formulations, drug delivery, and amine active ingredients. The effect of simple equipment and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
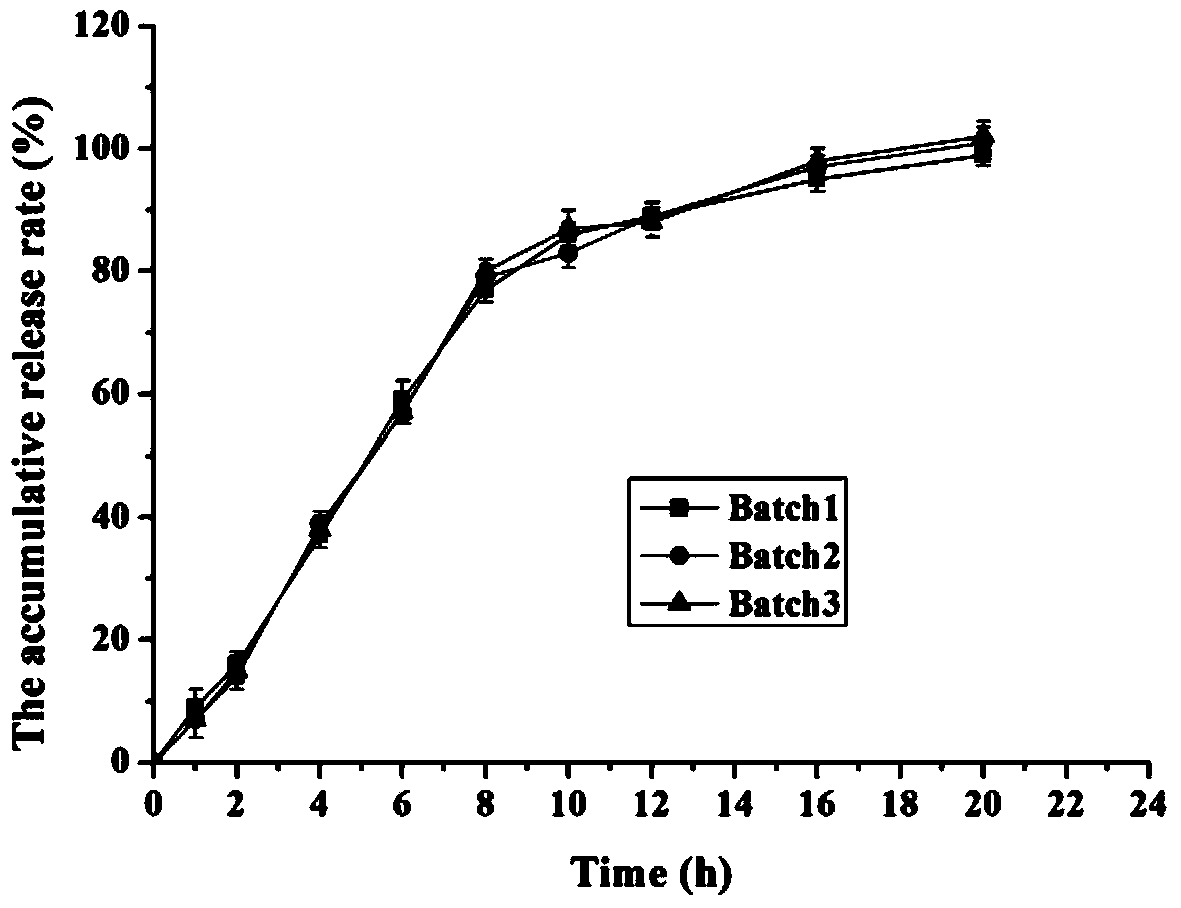
Embodiment 1
[0039] Tablet formulation: (made into 1000 tablets, each containing 28mg of memantine hydrochloride)
[0040]
[0041] The preparation method of the present embodiment memantine hydrochloride slow-release preparation comprises the steps:
[0042] (1) Memantine hydrochloride, skeleton material, diluent, lubricant, and binder are sieved separately, and then mixed evenly to obtain a mixed powder;
[0043] (2) Wet granulate the mixed powder and then press it with a 3mm punch to obtain tablet cores;
[0044] (3) Coat the tablet core with coating solution; the coating process parameters are: tablet bed weight 300g, coating pan rotation speed 5-35rpm, tablet bed temperature 25°C, coating solution inflow rate 4-5g / min, spray pressure 0.08-0.12MPa;
[0045] (4) Heat the coated finished product at 40°C for 12 hours to obtain memantine hydrochloride sustained-release mini-tablets;
[0046] (5) Put the memantine hydrochloride sustained-release mini tablets into hard capsules to obtai...
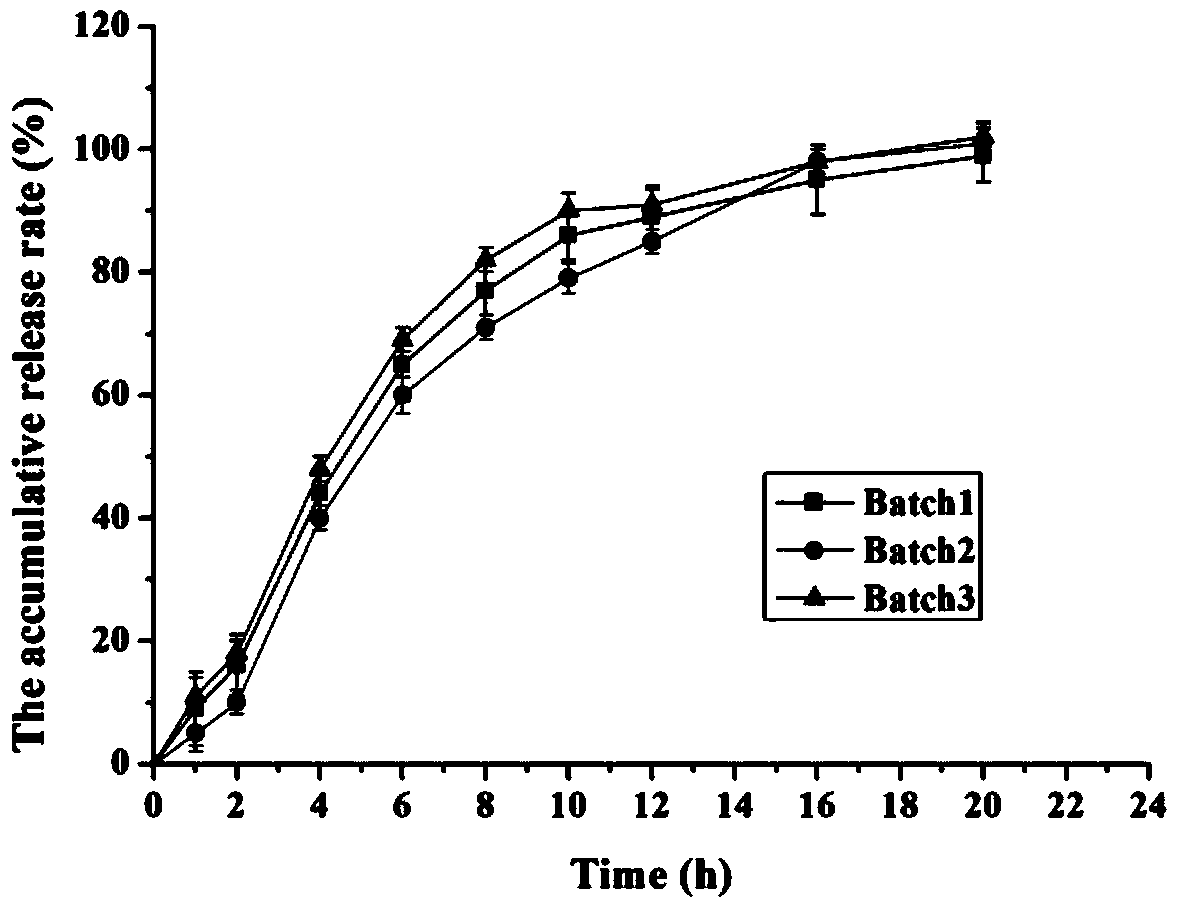
Embodiment 2
[0048] Tablet formula: (made into 1000 tablets, each containing 14mg of memantine hydrochloride)
[0049]
[0050]
[0051] The preparation method of the present embodiment memantine hydrochloride slow-release preparation comprises the steps:
[0052] (1) Memantine hydrochloride, skeleton material, diluent, lubricant, and binder are sieved separately, and then mixed evenly to obtain a mixed powder;
[0053] (2) Wet granulate the mixed powder and then press it with a 3mm punch to obtain tablet cores;
[0054] (3) Dissolve the coating material, porogen, and plasticizer in 95% ethanol solution to make a coating solution with a mass concentration of 7.5%, and add talcum powder to the above coating solution, stir evenly, To obtain a mixed coating solution;
[0055] (4) Coat the tablet core with the above mixed coating solution; the coating process parameters are: tablet bed weight 300g, coating pan speed 5-35rpm, tablet bed temperature 35°C, coating solution inflow rate 4- ...
Embodiment 3
[0059] Tablet formula: (make 1000 tablets, each tablet contains 7mg of memantine hydrochloride)
[0060]
[0061] The preparation method of the present embodiment memantine hydrochloride slow-release preparation comprises the steps:
[0062] (1) Memantine hydrochloride, skeleton material, diluent, lubricant, and binder are sieved separately, and then mixed evenly to obtain a mixed powder;
[0063] (2) Compress the mixed powder with a 2mm punch to obtain tablet cores;
[0064] (3) Dissolve the coating material, porogen, and plasticizer in 95% ethanol solution to make a coating solution with a mass concentration of 7.5%, and add talcum powder to the above coating solution, stir evenly, To obtain a mixed coating solution;
[0065] (4) Coat the tablet core with the above mixed coating solution; the coating process parameters are: tablet bed weight 300g, coating pan speed 5-35rpm, tablet bed temperature 50°C, coating solution inflow rate 4- 5g / min, spray pressure 0.08-0.12MPa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com