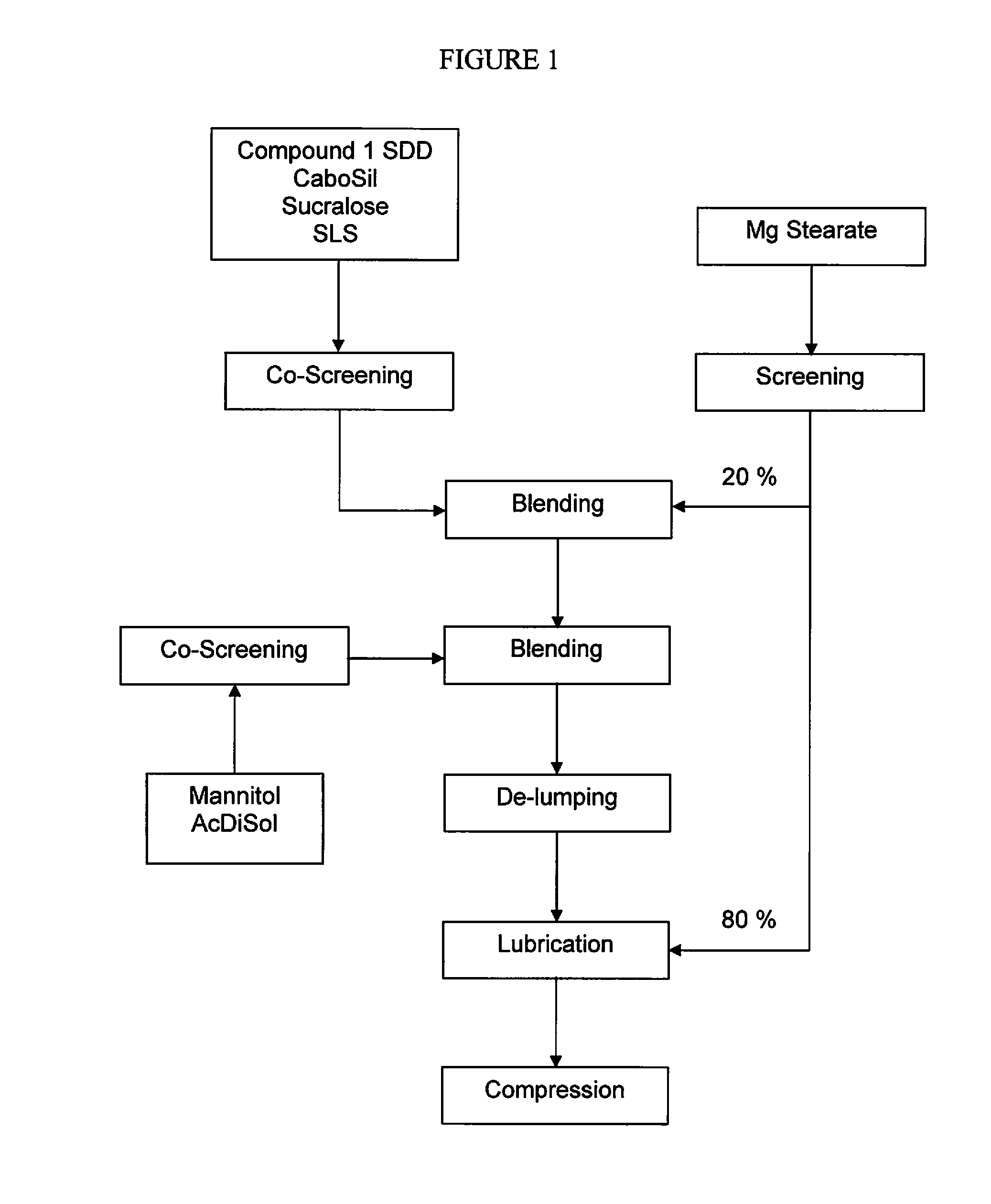
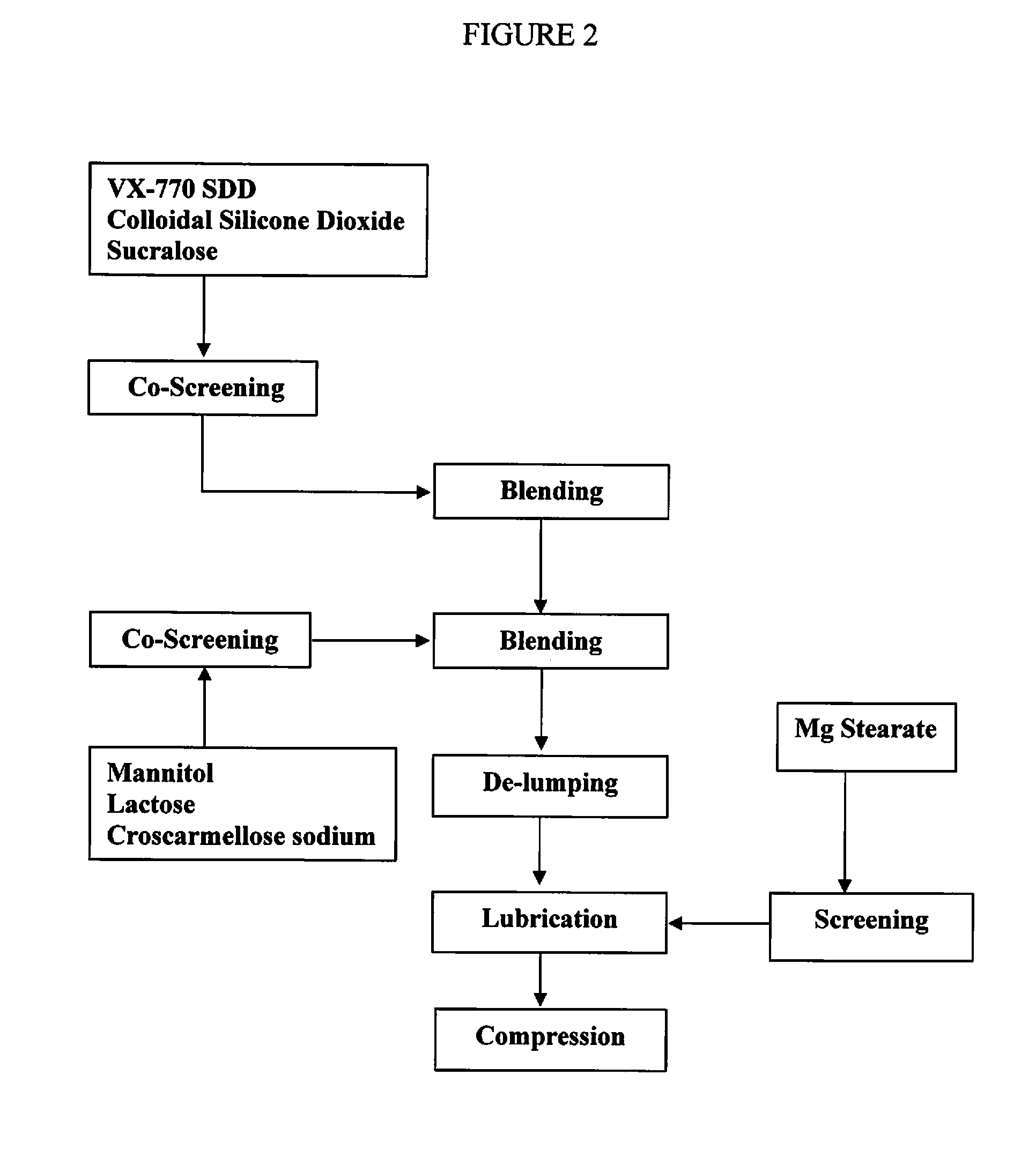
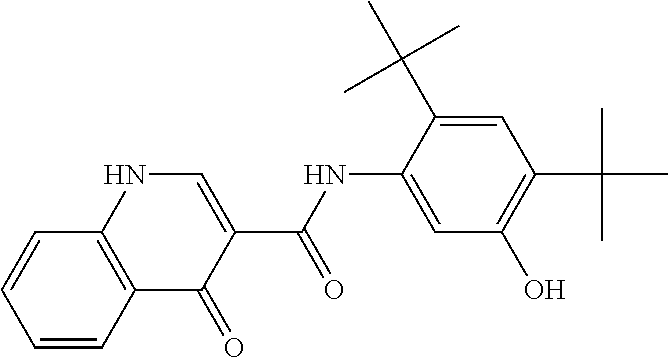
Pharmaceutical composition and administrations thereof
a technology of pharmaceutical compositions and compositions, applied in the field of pharmaceutical compositions, can solve the problems of imbalance in ion and fluid transport, no cure, and individuals with two copies of the cf associated gene suffering from the debilitating and fatal effects of cf, and achieve the effects of enhancing its visual appeal, taste, and scen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Manufacturing Intermediate 1 Containing Substantially Amorphous or Amorphous Compound 1
[1693]The synthesis of N-[2,4-Bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide is described in United States patent application publication numbers US 2006 / 0074075 (now U.S. Pat. No. 7,495,103), US 2011 / 0064811, US 2010 / 0267768, and US 2011 / 0230519, the contents of which are hereby incorporated by reference in their entirety. A solvent system of MEK and DI water, formulated according to the ratio 90 wt % MEK / 10 wt % DI water, was heated to a temperature of 20-30° C. in a reactor, equipped with a magnetic stirrer and thermal circuit. Into this solvent system, hypromellose acetate succinate polymer (HPMCAS)(HG grade), SLS, and N-[2,4-Bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide were added according to the ratio 19.5 wt % hypromellose acetate succinate / 0.5 wt % SLS / 80 wt % N-[2,4-Bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-...
example 2
Manufacturing a Powder Blend Containing about 75 mg of Substantially Amorphous or Amorphous Compound 1 Encapsulated in Exemplary Capsule 1
[1698]A batch of powder blend is formulated for encapsulation to have approximately 75 mg of Compound 1 per capsule using the amounts of ingredients recited in Table 2.
TABLE 2Ingredients for Exemplary Capsule 1 Containing a Powder Blend.Percent DoseDoseBatchFormulation% Wt. / Wt.(mg)(g)Intermediate 146.9%95.2952Mannitol49.1%99.7997Sucralose 2.0%4.141Colloidal silicon dioxide 1.0%2.020Magnesium stearate 1.0%2.030Total 100%2032030
[1699]Intermediate 1, mannitol (Pearlitol® 100 SD commercially available from Roquette America Inc. of Keokuk IA), sucralose (Splenda® commercially available from Tate and Lyle of Decatur, Ill.), colloidal silicon dioxide (Cabot Cab-O-Sil® M-5P Fumed Silicon Dioxide, commercially available from Cabot Corporation of Alpharetta, Ga.) and magnesium stearate (Fisher Scientific or as Hyqual®, commercially available from Mallinckro...
example 3
Manufacturing a Powder Blend Containing about 75 mg of Substantially Amorphous or Amorphous Compound 1 Encapsulated in Exemplary Capsule 2
[1701]A batch of powder blend was formulated for encapsulation to have approximately 75 mg of Compound 1 per capsule using the amounts of ingredients recited in Table 3.
TABLE 3Ingredients for Exemplary Capsule 2 Containing a Powder Blend.Percent DoseDoseBatchFormulation% Wt. / Wt.(mg)(g)Intermediate 146.9%93.8469.07Mannitol49.1%98.2491.17Sucralose 2.0%4.020.01Colloidal silicon dioxide 1.0%2.010.02Magnesium stearate 1.0%2.010.03Total100%2001000.3
[1702]Intermediate 1 and Sucralose (commercially available from Tate and Lyle of Decatur, Ill.) were co-screened through 20 mesh (850 micrometer) screen. Mannitol (Pearlitol® 100 SD commercially available from Roquette America Inc. of Keokuk Iowa) and colloidal silicon dioxide (Cabot Cab-O-Sil® M-5P Fumed Silicon Dioxide, commercially available from Cabot Corporation of Alpharetta, Ga.) were co-screened throu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| mean particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com