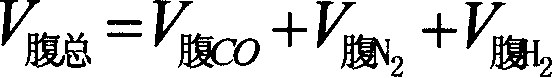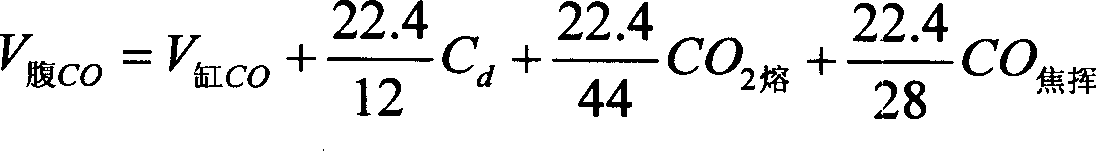Patents
Literature
96 results about "Equilibrium constant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further change. For a given set of reaction conditions, the equilibrium constant is independent of the initial analytical concentrations of the reactant and product species in the mixture. Thus, given the initial composition of a system, known equilibrium constant values can be used to determine the composition of the system at equilibrium. However, reaction parameters like temperature, solvent, and ionic strength may all influence the value of the equilibrium constant.
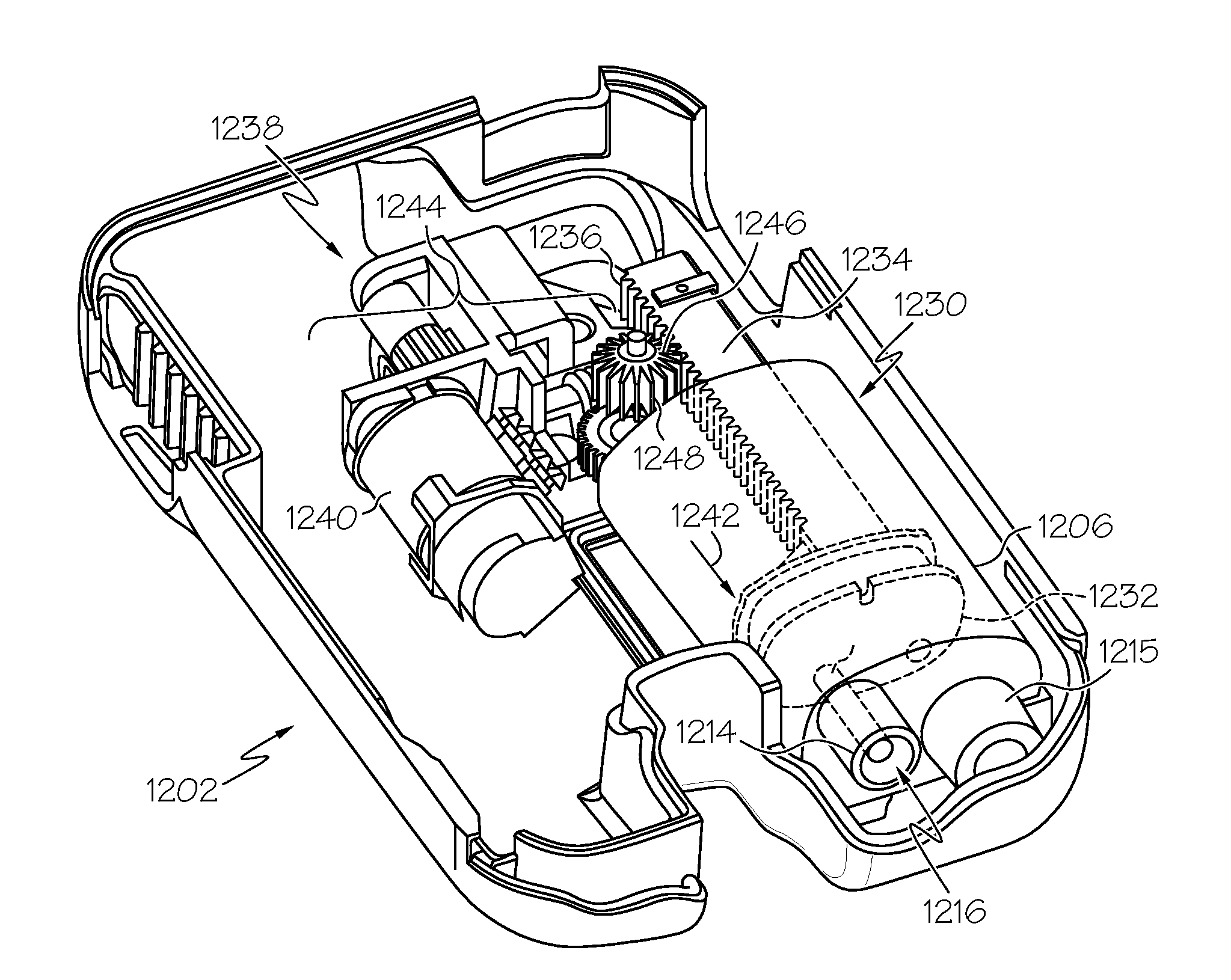
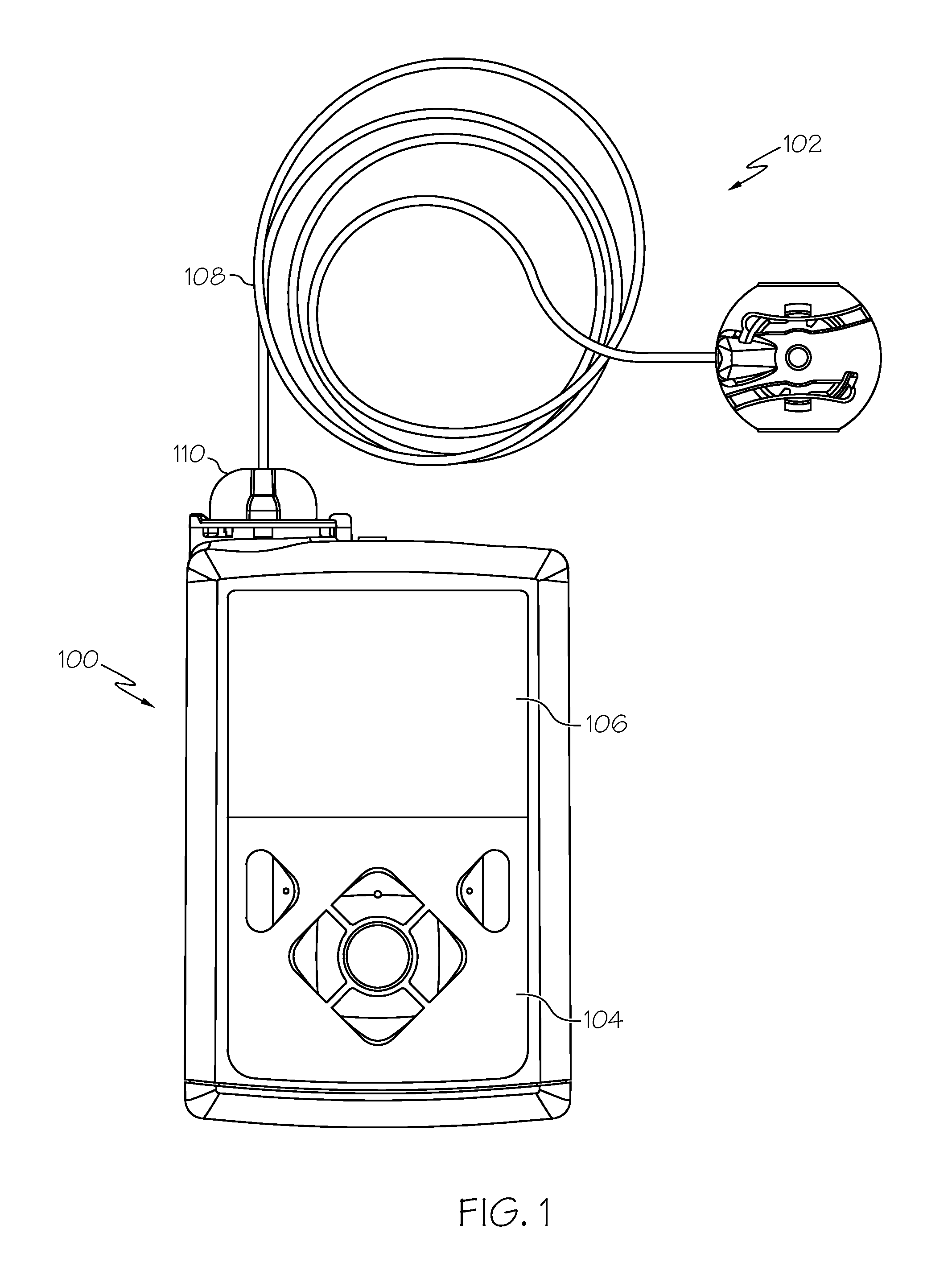
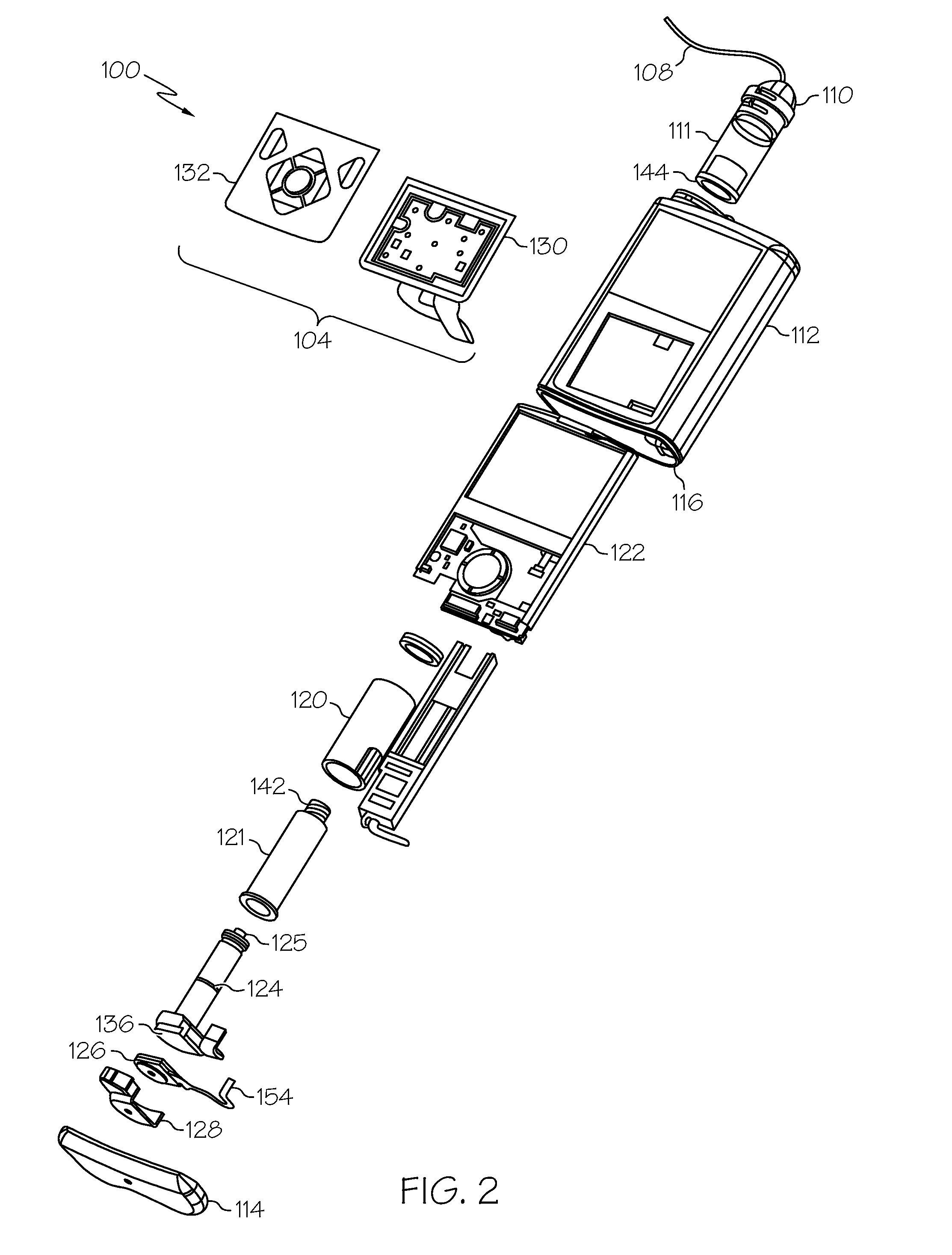
Fluid reservoir seating procedure for a fluid infusion device
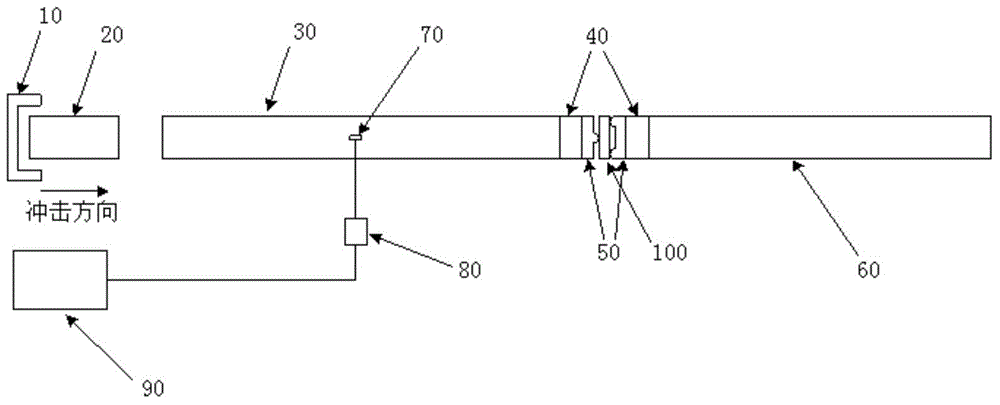
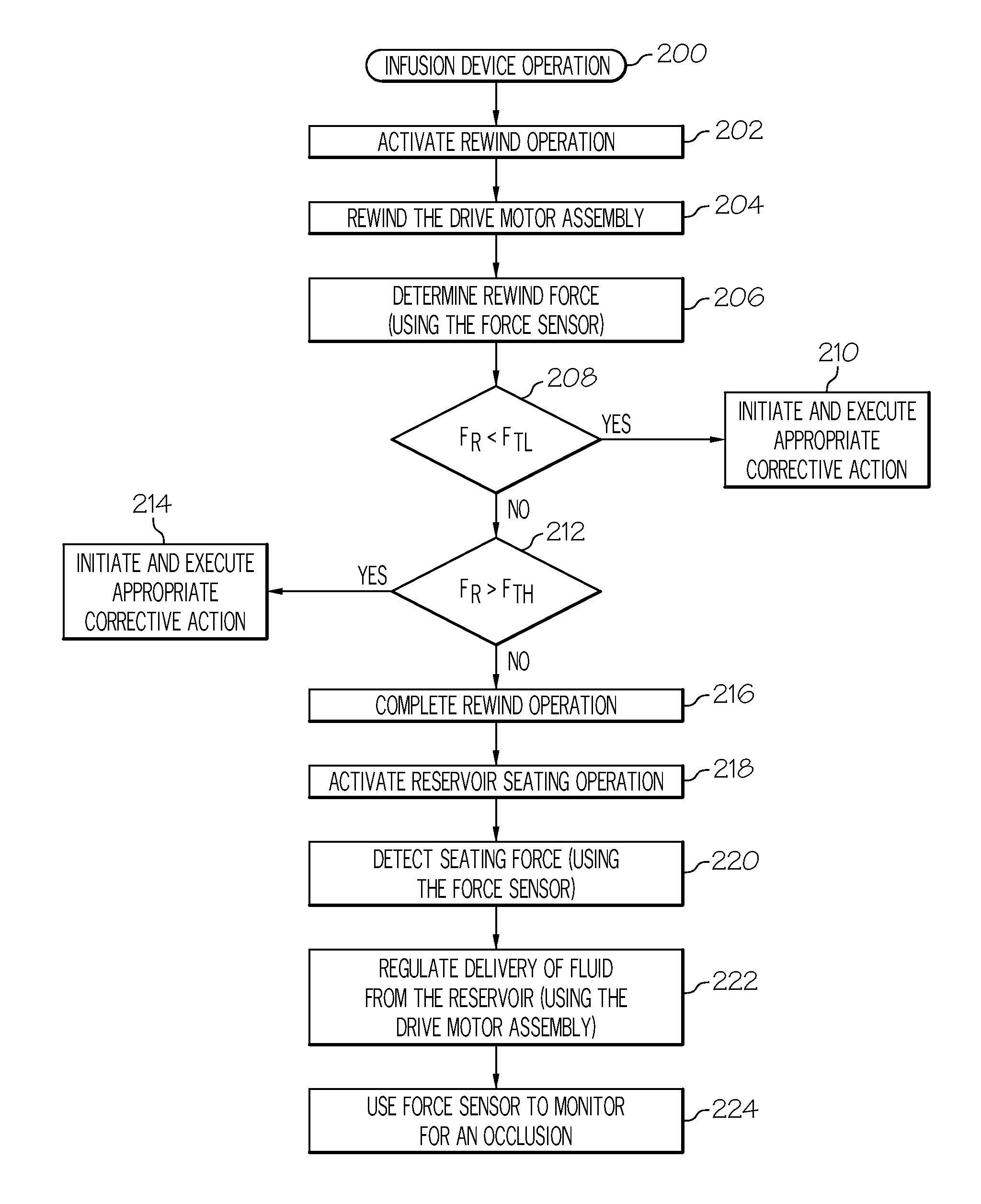
A method of seating a fluid reservoir in a housing of a fluid infusion device is presented here. The method is performed prior to establishing an outgoing fluid flow path from the fluid reservoir. The method begins by detecting insertion of the fluid reservoir into the housing of the fluid infusion device. In response to detecting the insertion, the method determines whether the fluid reservoir is in need of depressurization. When the fluid reservoir is in need of depressurization, the drive motor assembly of the fluid infusion device is rewound to depressurize the fluid reservoir. After depressurizing the fluid reservoir, an equalization state for the fluid reservoir is achieved. After achieving the equilibrium state, the drive motor assembly is advanced to obtain an initial seated state for the fluid reservoir.
Owner:MEDTRONIC MIMIMED INC
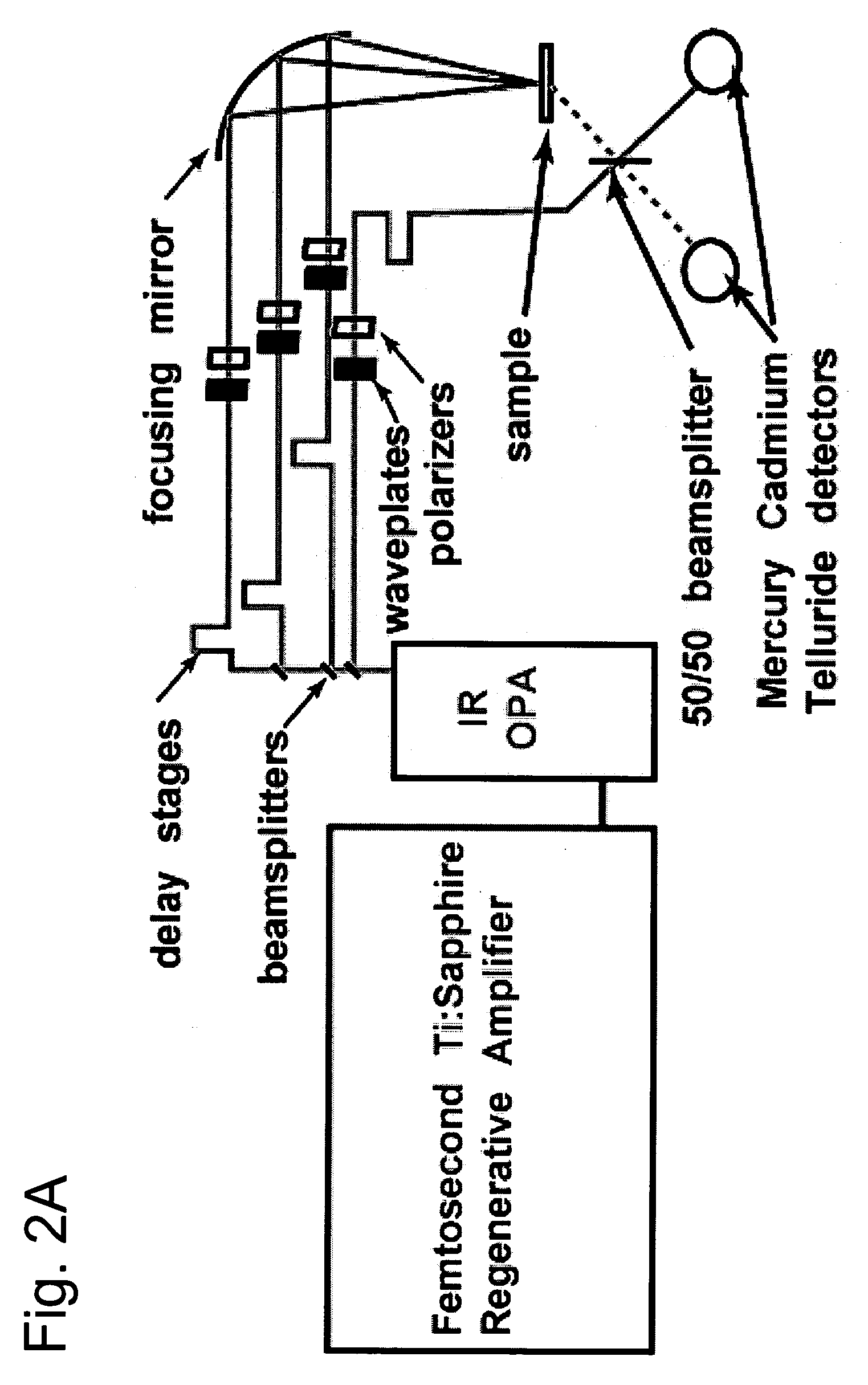
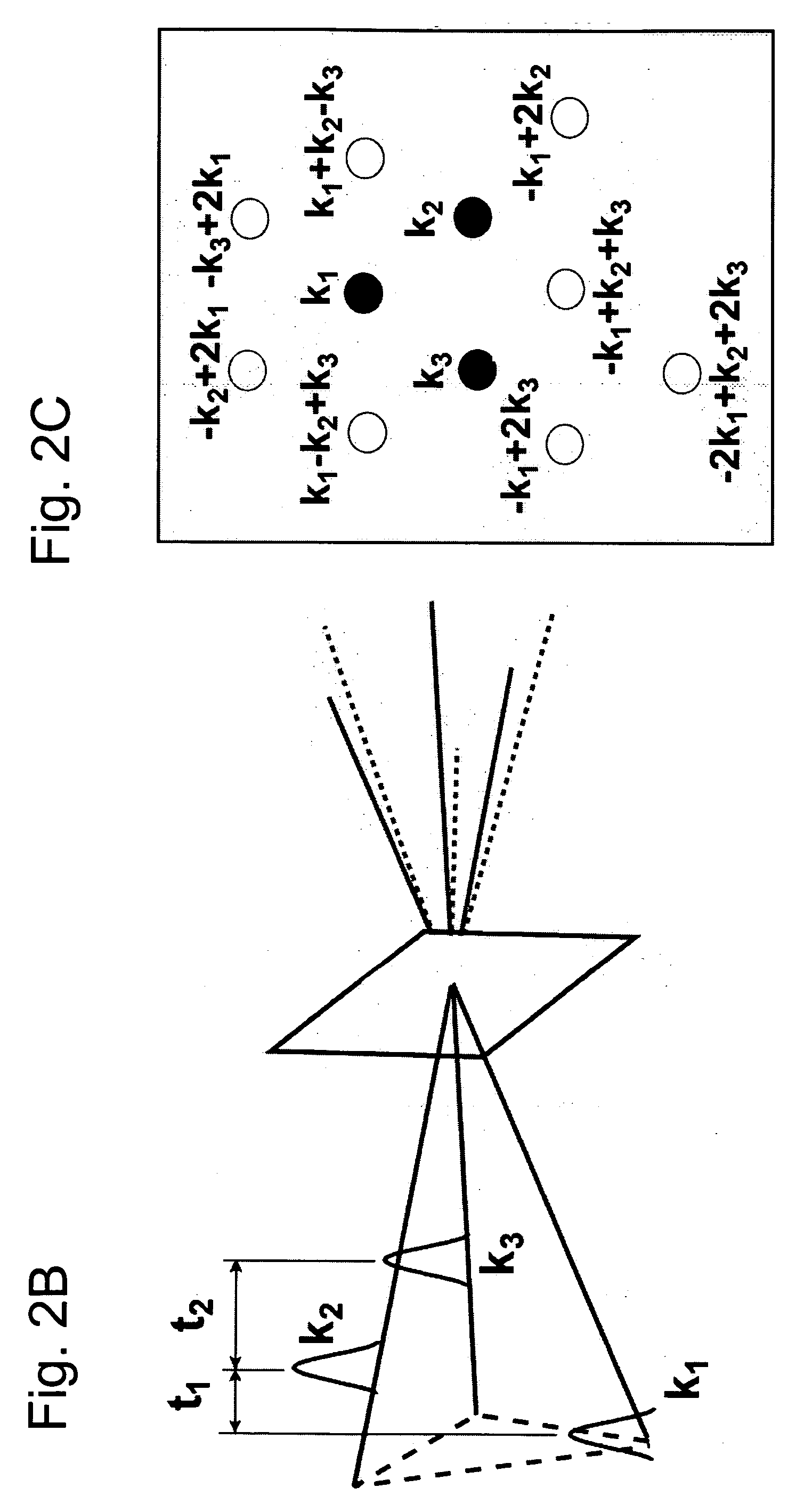
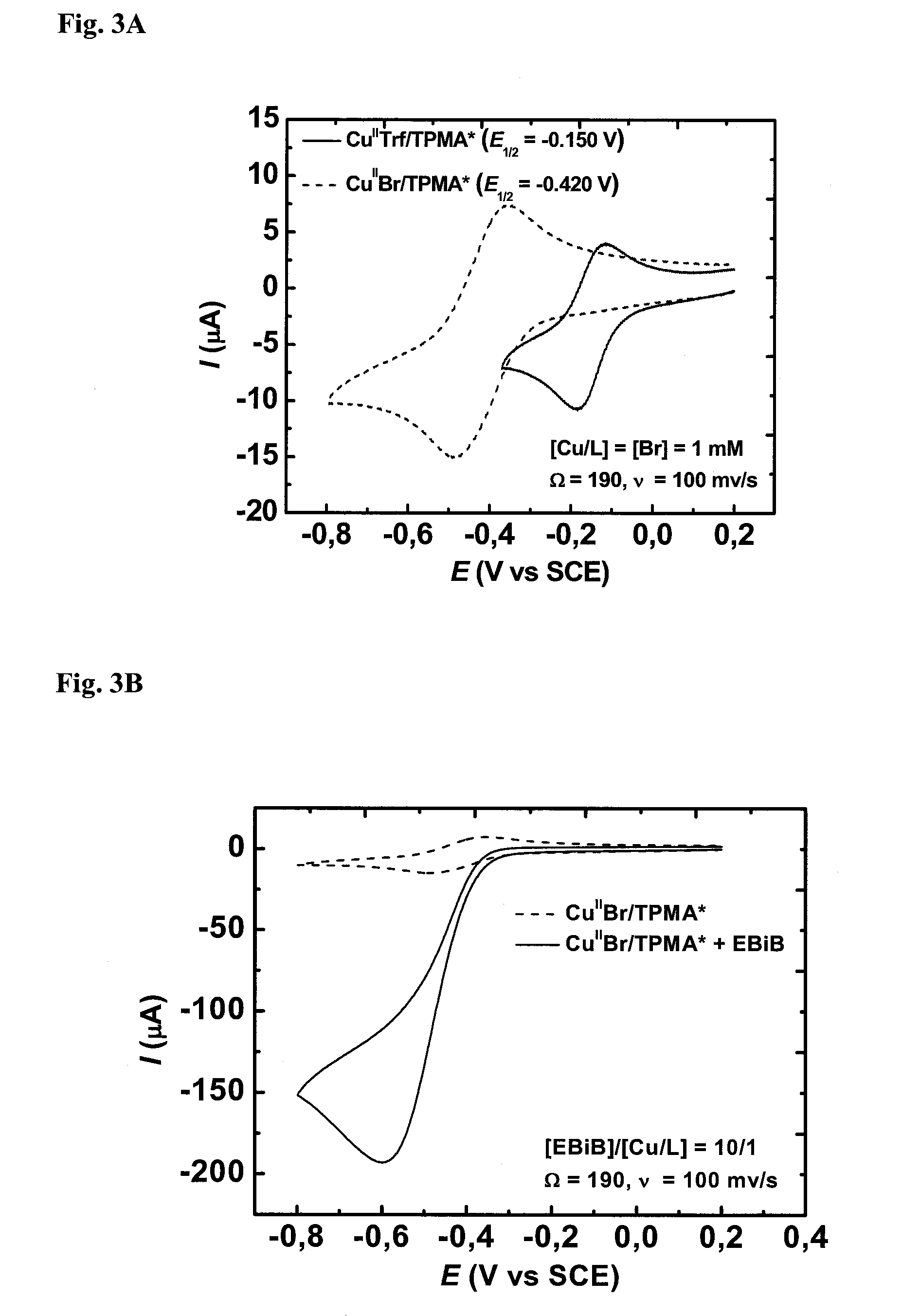
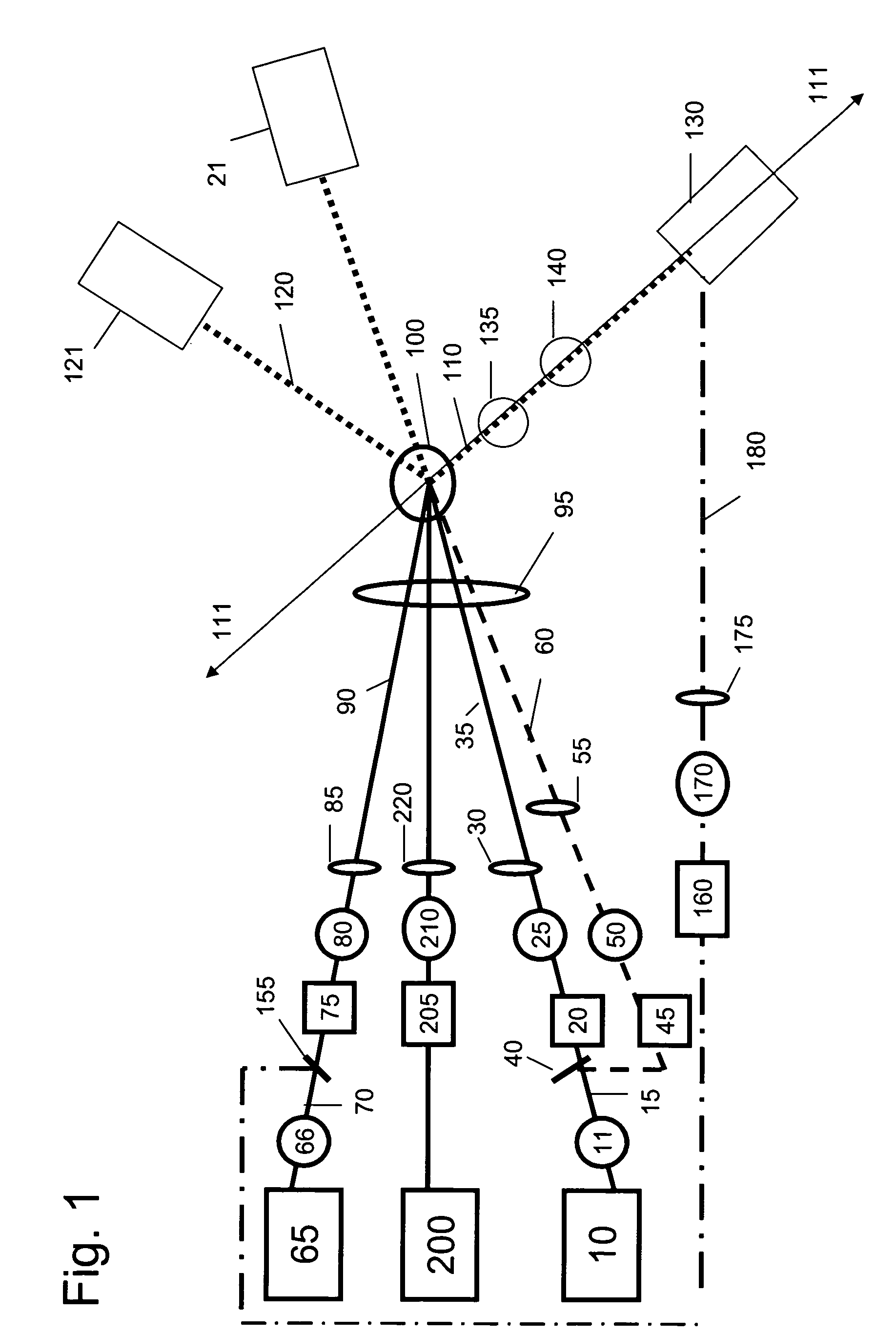
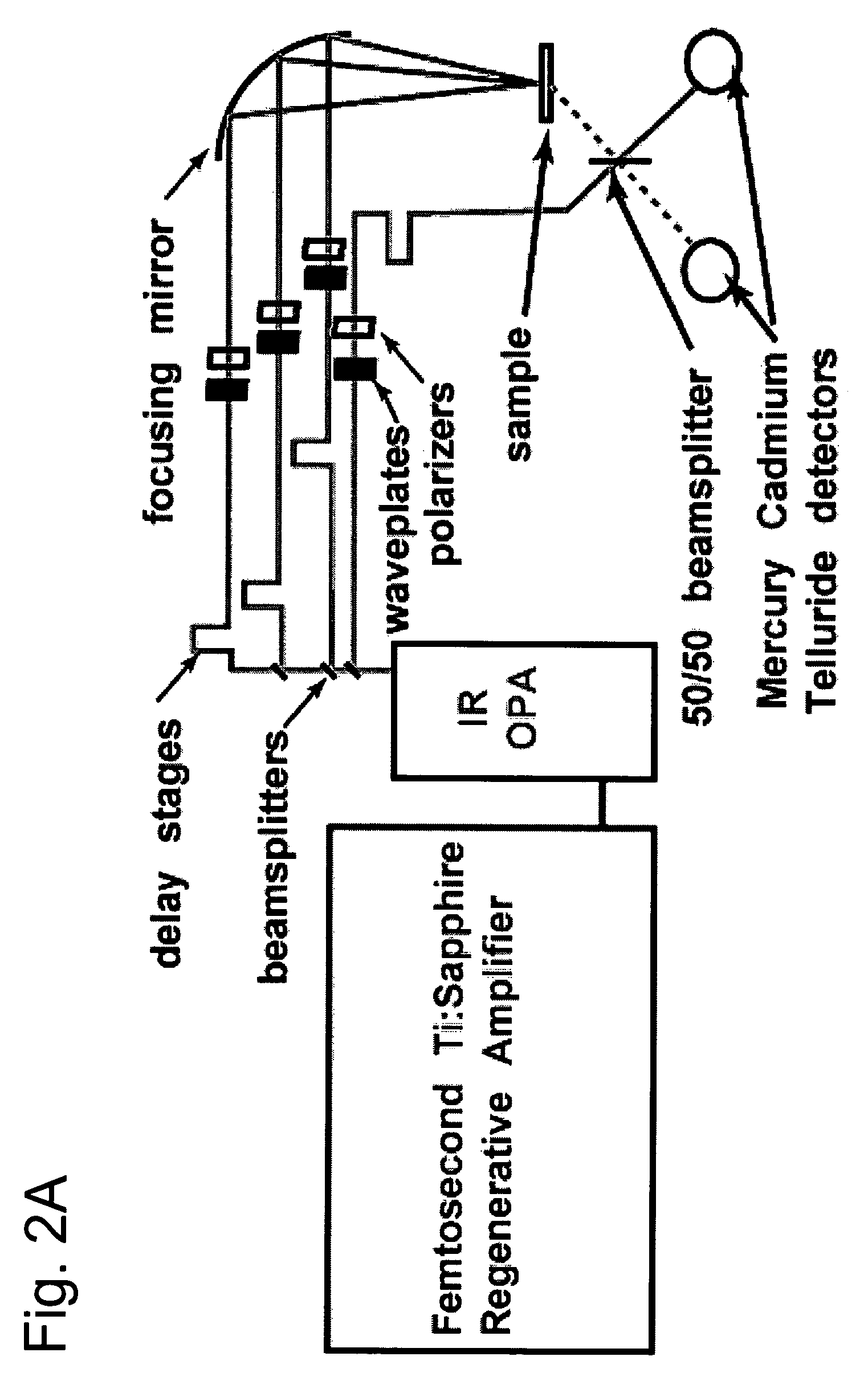
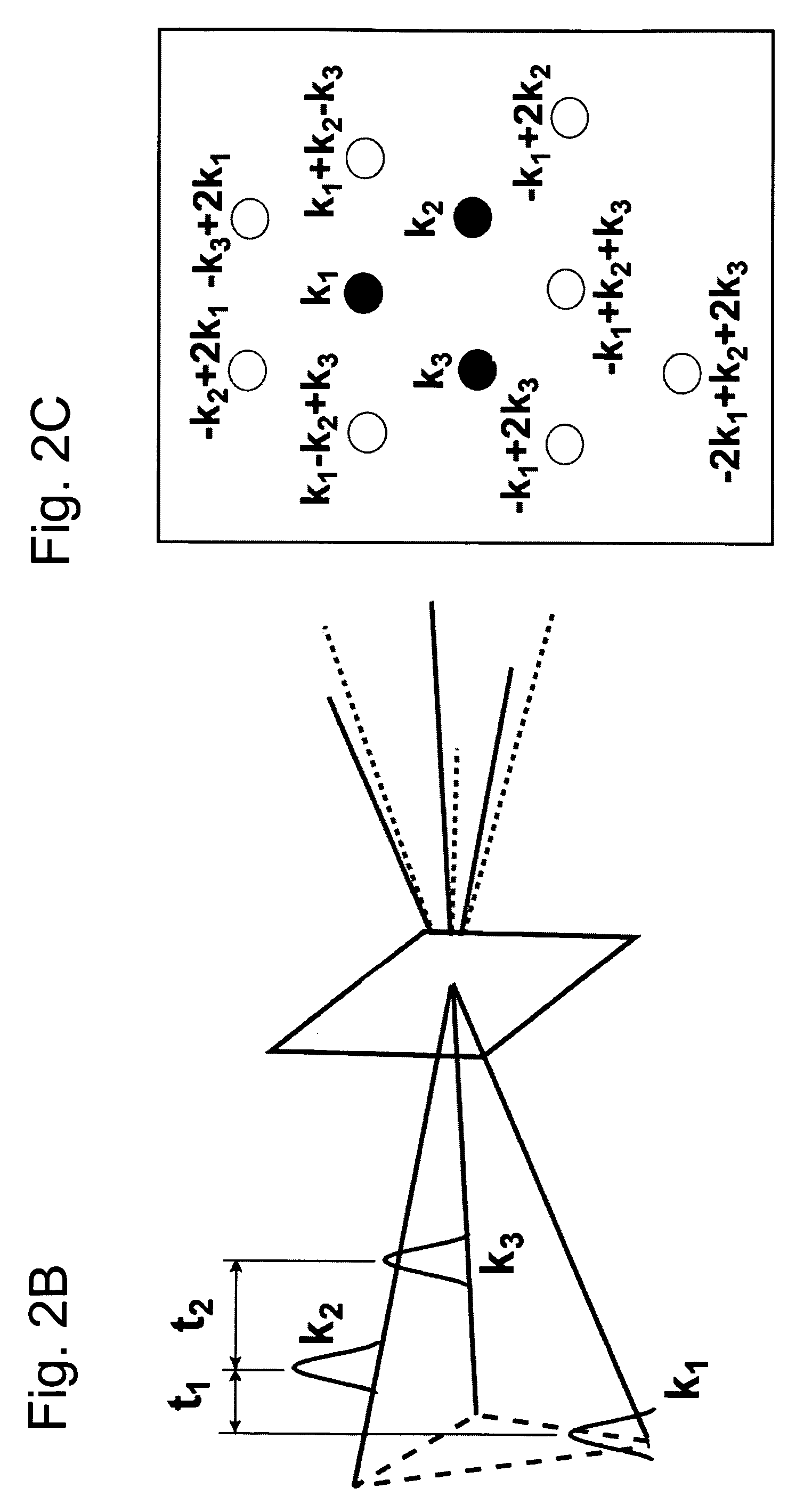
Nonlinear spectroscopic methods for identifying and characterizing molecular interactions
ActiveUS20060063188A1Remarkable effectSmall structureSugar derivativesPeptide/protein ingredientsNonlinear spectroscopyEquilibrium constant
This invention provides methods and devices for identifying and / or characterizing interactions involving molecules, including, but not limited to, identifying and / or characterizing interactions involving target molecules and candidate molecules. The present invention provides methods using multidimensional infrared spectrographic techniques, such as four wave mixing and pump-probe techniques, for identifying interactions involving biomolecules and therapeutic candidate molecules, and for characterizing such interactions in terms of their binding coefficients and / or equilibrium constants.
Owner:WISCONSIN ALUMNI RES FOUND
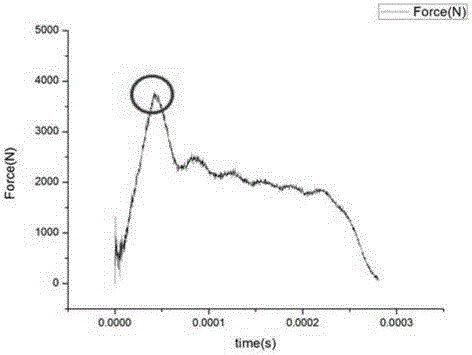
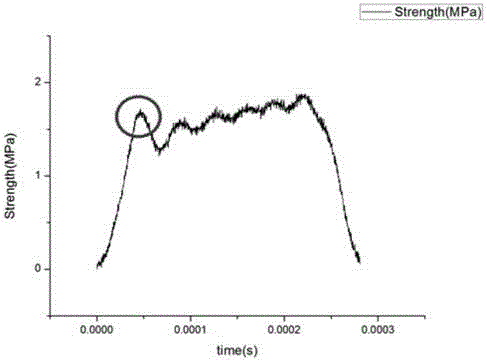
Determination apparatus and determination method for concrete impact flexural-tensile injury based on non-equilibrium state
InactiveCN104913985AOvercoming the difficulty of measuring the transmitted wave in the testReduce mistakesMaterial strength using single impulsive forceData acquisitionEquilibrium constant
The present invention discloses a determination apparatus for concrete impact flexural-tensile injury based on non-equilibrium state. The determination apparatus is characterized by comprising a gas gun, a bullet, an incident rod, a transmission rod, a strain sheet, a bridge box and a data acquisition system connected with a computer, wherein the strain sheet is adhered on the middle portion of the incident rod and is connected with the data acquisition system through the bridge box, the incident rod and the transmission rod are respectively provided with a sleeve head, the sleeve heads are respectively provided with a pad block, and a concrete specimen is sandwiched between the pad blocks on the two rods. With the determination apparatus and the determination method of the present invention, the concrete injury theory is introduced into the flexural concrete determination under impact load, such that the difficulty that the flexural concrete injury time is short under the impact load is overcome, and the relationship curve between the tensile strength and the injury time during the concrete flexibility is obtained; and the difficult problem that the transmitted wave is difficult to determine through the test is overcome, and the error is substantially reduced during the wave superimposing.
Owner:POWERCHINA XIBEI ENG +1
Fluid reservoir seating procedure for a fluid infusion device
A method of seating a fluid reservoir in a housing of a fluid infusion device is presented here. The method is performed prior to establishing an outgoing fluid flow path from the fluid reservoir. The method begins by detecting insertion of the fluid reservoir into the housing of the fluid infusion device. In response to detecting the insertion, the method determines whether the fluid reservoir is in need of depressurization. When the fluid reservoir is in need of depressurization, the drive motor assembly of the fluid infusion device is rewound to depressurize the fluid reservoir. After depressurizing the fluid reservoir, an equalization state for the fluid reservoir is achieved. After achieving the equilibrium state, the drive motor assembly is advanced to obtain an initial seated state for the fluid reservoir.
Owner:MEDTRONIC MIMIMED INC
Contact lens with a hydrophilic layer
ActiveUS20150234204A1Adequate on eye movementStay healthySpectales/gogglesOptical articlesRigid gas permeable lensHydrophilic polymers
Embodiments of the technology relate to a contact lens having a core that is coated by a hydrogel layer, and to methods of making such a lens. The coated lens can include a rigid gas permeable contact lens. The coated lens can also include a hybrid silicone and rigid gas permeable contact lens. In one aspect, embodiments provide for a coated contact lens comprising a lens core with a water equilibrium constant of less than about 2% comprising an outer surface; and a hydrogel layer covalently attached to at least a portion of the outer surface, the hydrogel layer adapted to contact an ophthalmic surface, wherein the hydrogel layer comprises a hydrophilic polymer population of one or more species.
Owner:TANGIBLE SCI LLC
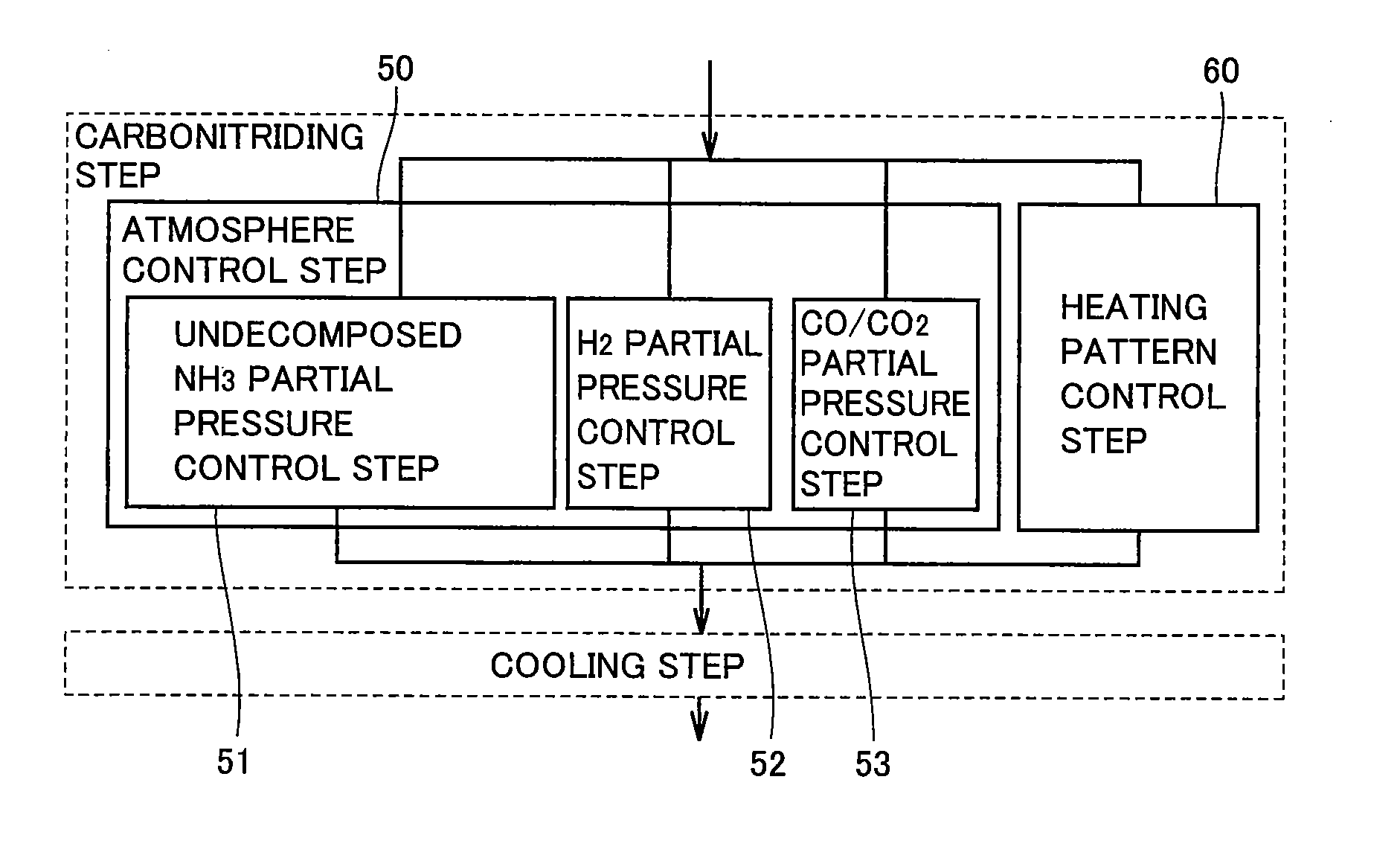
Carbonitriding method, machinery component fabrication method, and machinery component
ActiveUS20100154937A1Improves nitrogen permeating rateReduce manufacturing costBearing componentsSolid state diffusion coatingNitrogenEngineering
A carbonitriding method that can improve the nitrogen permeating rate to render the carbonitriding process effective includes an atmosphere control step (50), and a heating pattern control step (60). The atmosphere control step (50) includes an undecomposed NH3 partial pressure control step (51), and a CO / CO2 partial pressure control step (53). The undecomposed NH3 partial pressure control step (51) and the CO / CO2 partial pressure control step (53) are carried out in the atmosphere control step (50) such that ac* defined by the following equation (1) is at least 0.88 and not more than 1.27, and α defined by equation (2) is at least 0.012 and not more than 0.020, where PN is the undecomposed ammonia partial pressure and PH is the hydrogen partial pressure in the heat treatment furnace, whereinac*=(Pco)2K×Pco2(1)PCO: partial pressure of carbon dioxide (atm), PCO<sub2>2< / sub2>: partial pressure of carbon dioxide (atm)K: equlibrium constant at <C>+CO22COα=PN0.006×(PH)32×(1.877-1.055×ac*)100(2)
Owner:NTN CORP
Treatment method for rare earth phosphate rock and enrichment method for rare earth
ActiveCN103184356AReduce eutectic adsorptionEasy accessProcess efficiency improvementRare-earth elementEnrichment methods
The invention discloses a treatment method for a rare earth phosphate rock and an enrichment method for rare earth. The treatment method comprises the following steps: mixing the rare earth phosphate rock and phosphoric acid to form mixed slurry; adding concentrated sulfuric acid into the mixed slurry in such a manner that the concentration of Ca<2+> in the mixed slurry is decreased from at least 1 wt% to the equilibrium concentration of Ca<2+> and SO4<2->, then the concentration of SO4<2-> in the mixed slurry is increased from the equilibrium concentration of Ca<2+> and SO4<2-> to at least 2 wt% and the mixed slurry is added during addition of the concentrated sulfuric acid to allow the rare earth phosphate rock to be dissolved so as to obtain hemihydrate gypsum; and subjecting the obtained hemihydrate gypsum to recrystallization to obtain dihydrate gypsum. The enrichment method for rare earth comprises a step of recovering rare earth elements from liquid obtained after recrystallization of the hemihydrate gypsum and / or liquid obtained after heating of the mixed slurry. In the process of generation of the hemihydrate gypsum, the addition speed of the concentrated sulfuric acid is controlled, and SO4<2-> in the mixed slurry is allowed to be insufficient at first and then controlled to be excess, which aids rare earth in entering into the phosphoric acid.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Power tool level indicator
InactiveUS7154406B1Easy to useEasy to buildAcceleration measurementElectric switchesElectricityLeveling mechanism
A power tool includes a leveling mechanism that determines horizontal or vertical planes of the power tool. The leveling mechanism has a housing with a cavity in the housing. A rotating member is in the housing. The rotating member moves in the cavity such that the rotating members seeks an equilibrium position. The equilibrium position corresponds to a horizontal or vertical plane. Electrical contacts are coupled with the rotating member such that the electrical contacts only complete an electrical circuit when the rotating member is in the equilibrium position. An indicator is electrically coupled with the electrical contacts to indicate to the user when the mechanism is in an equilibrium position. A power source is coupled with the electrical contacts and the indicator to energize the indicator when the circuit is complete.
Owner:BLACK & DECKER INC
Measurement of concentrations and binding energetics
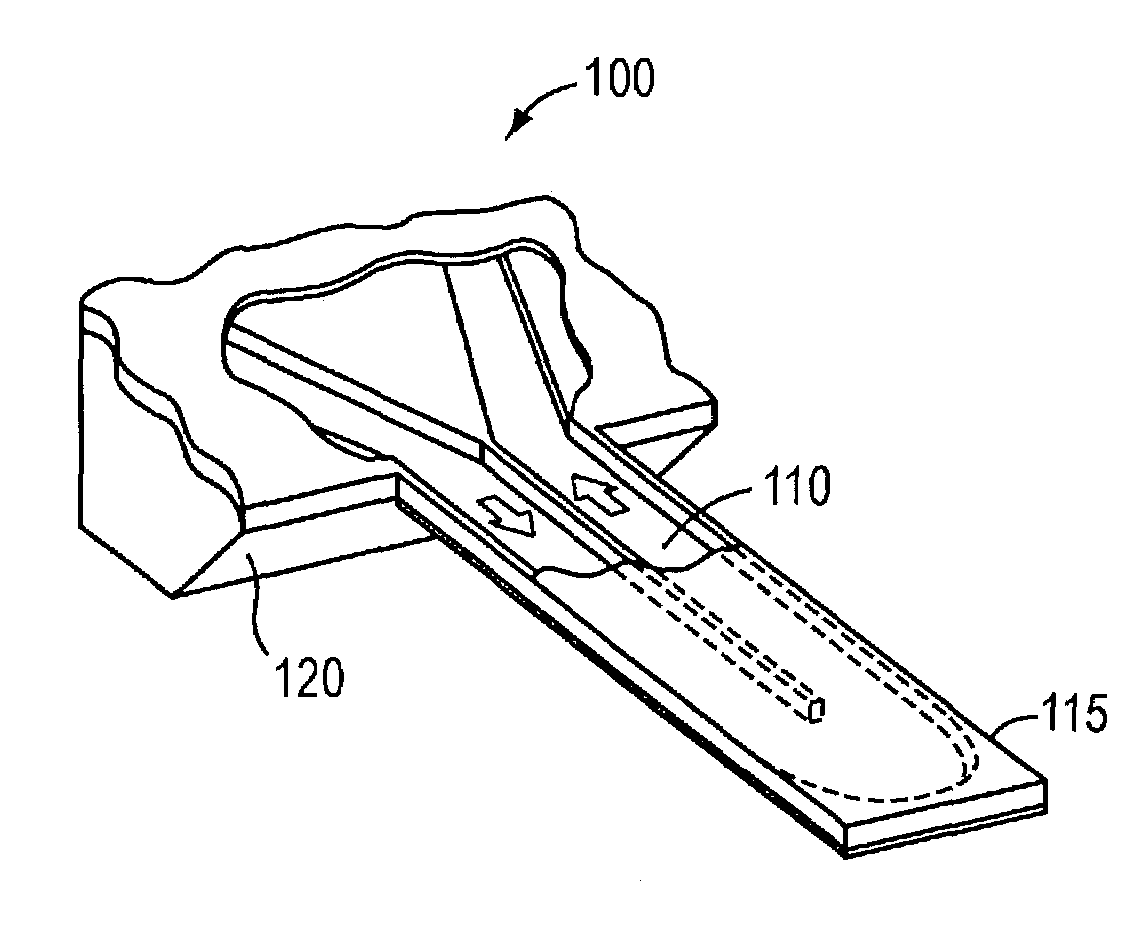
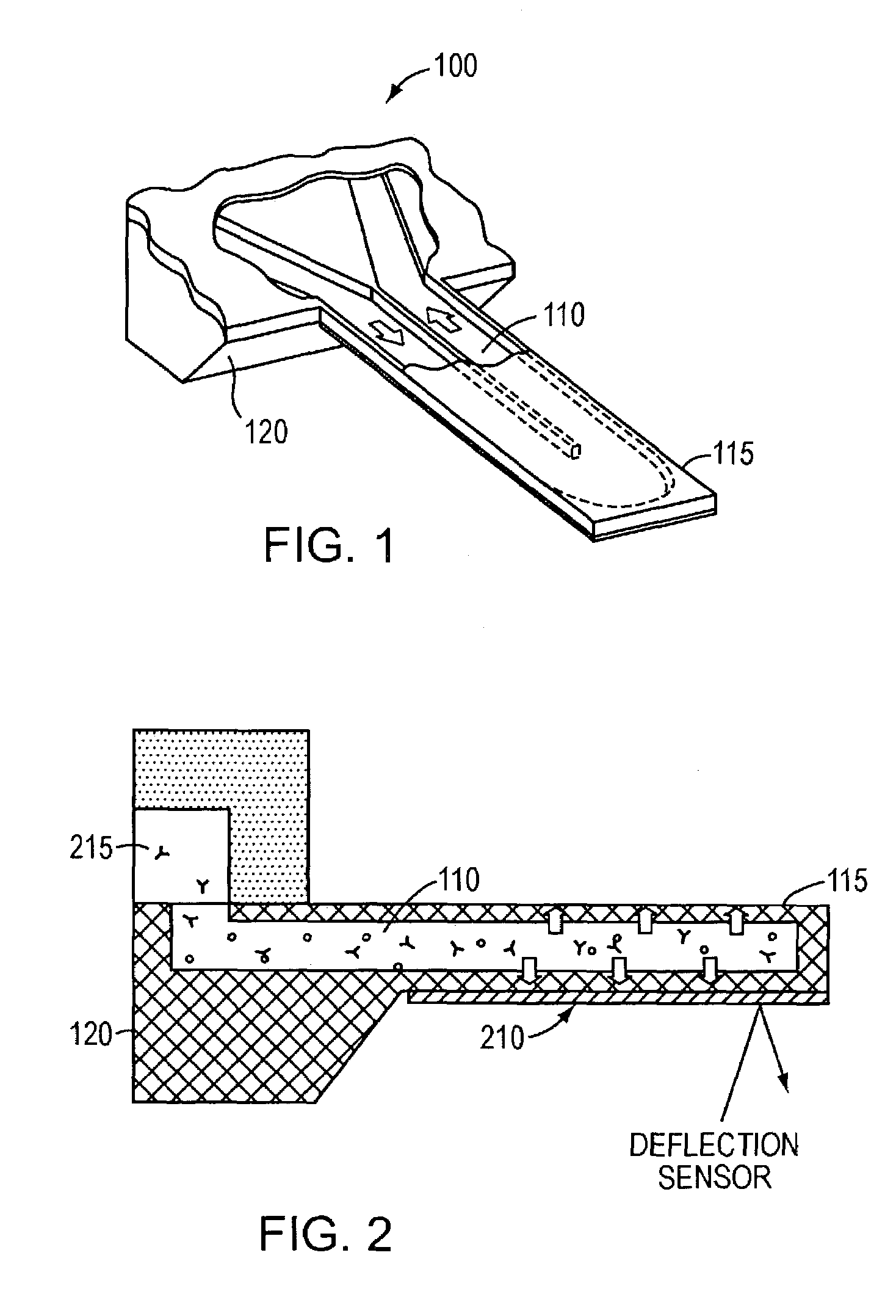
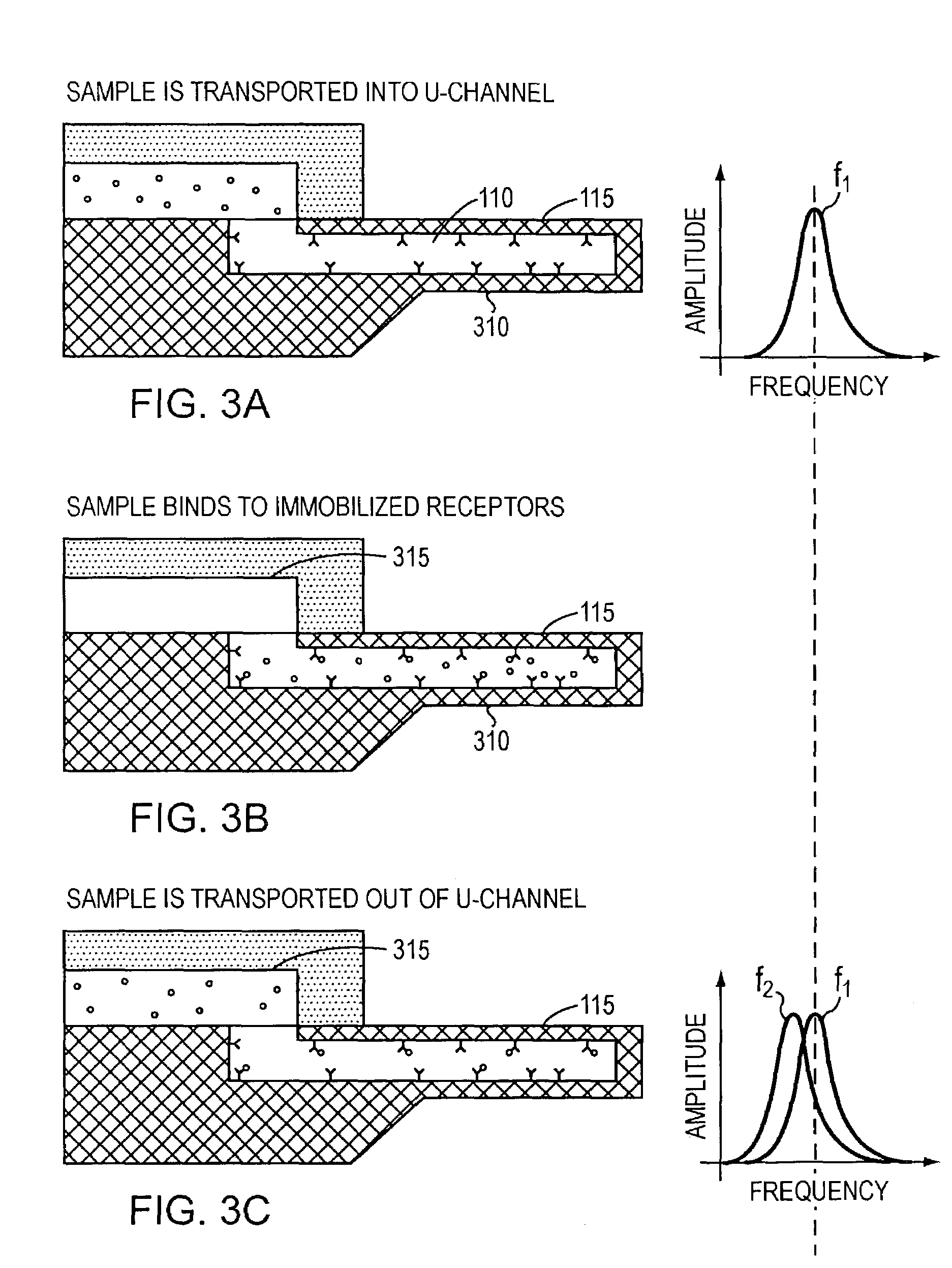
ActiveUS7387889B2Eliminate needBioreactor/fermenter combinationsBiological substance pretreatmentsEnergeticsEquilibrium constant
Free-standing microfluidic channels are used to both transport and analyze molecules of interest. In a biochemical context, such molecules may be polypeptides, nucleic acids, or other biomolecules. The free-standing channels provide a real-time readout of concentration without the need for labeling with reporter molecules. The channels can also measure enthalpy values and equilibrium constants by detecting heat released from or absorbed by the sample.
Owner:MASSACHUSETTS INST OF TECH
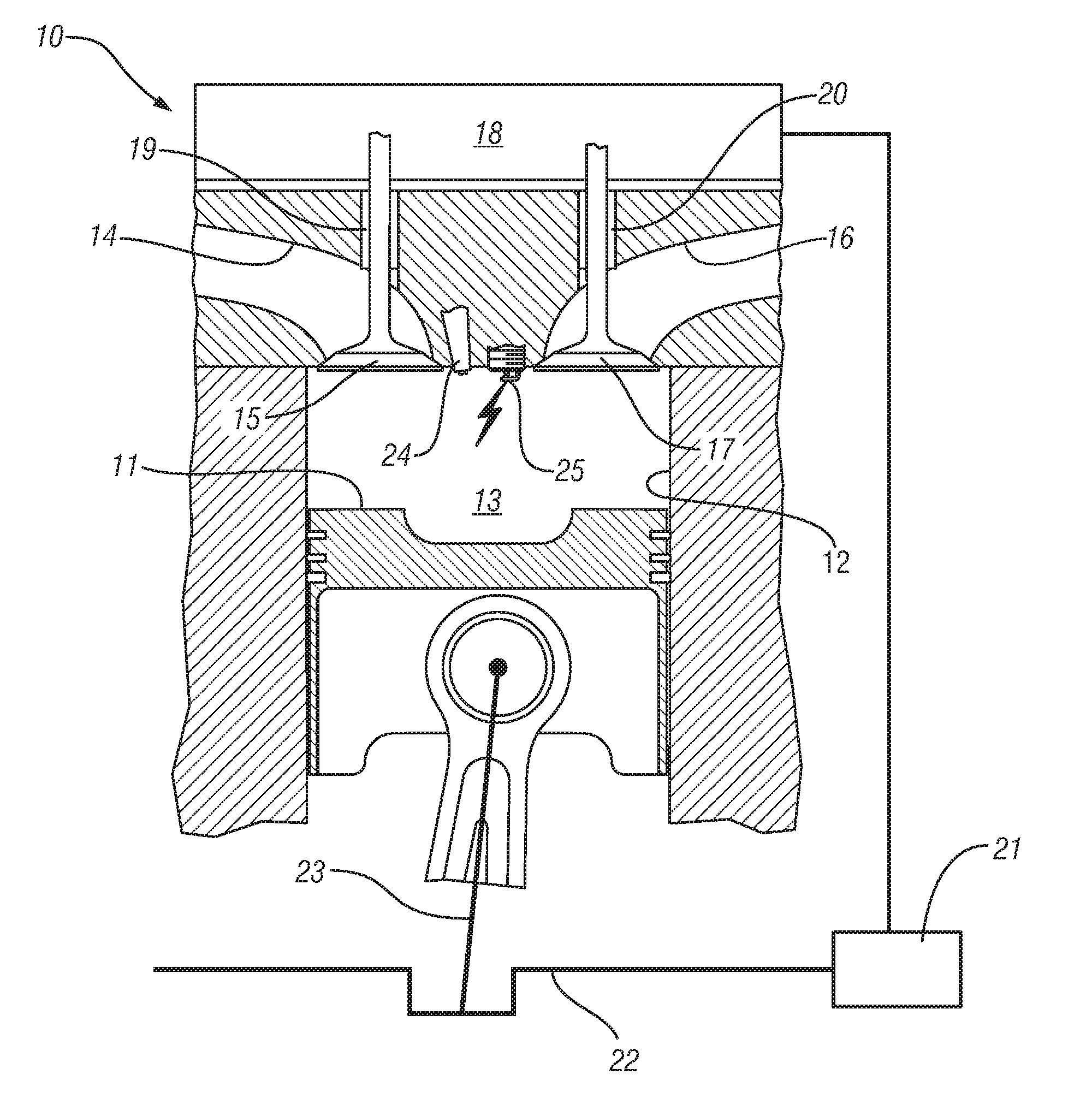
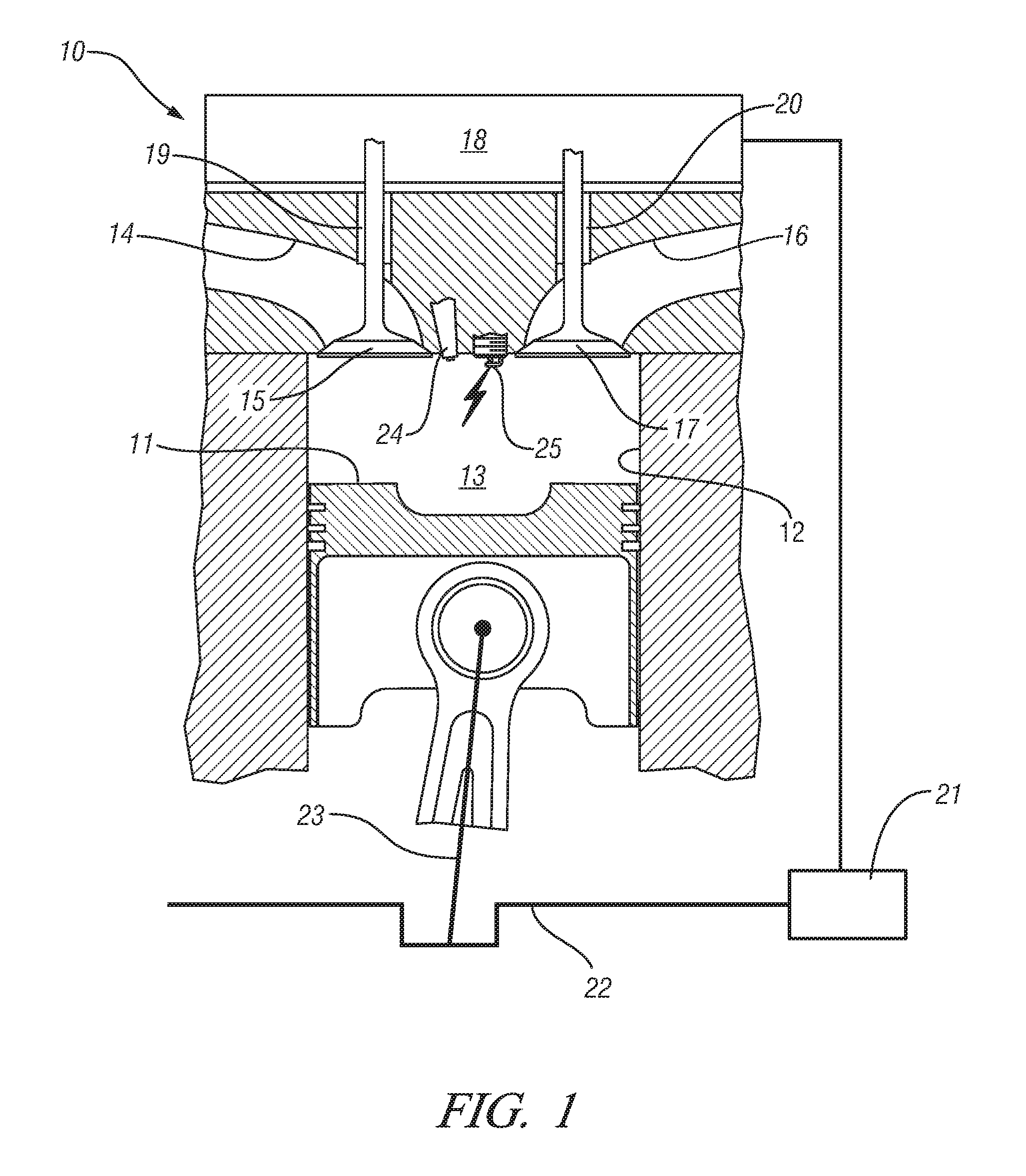
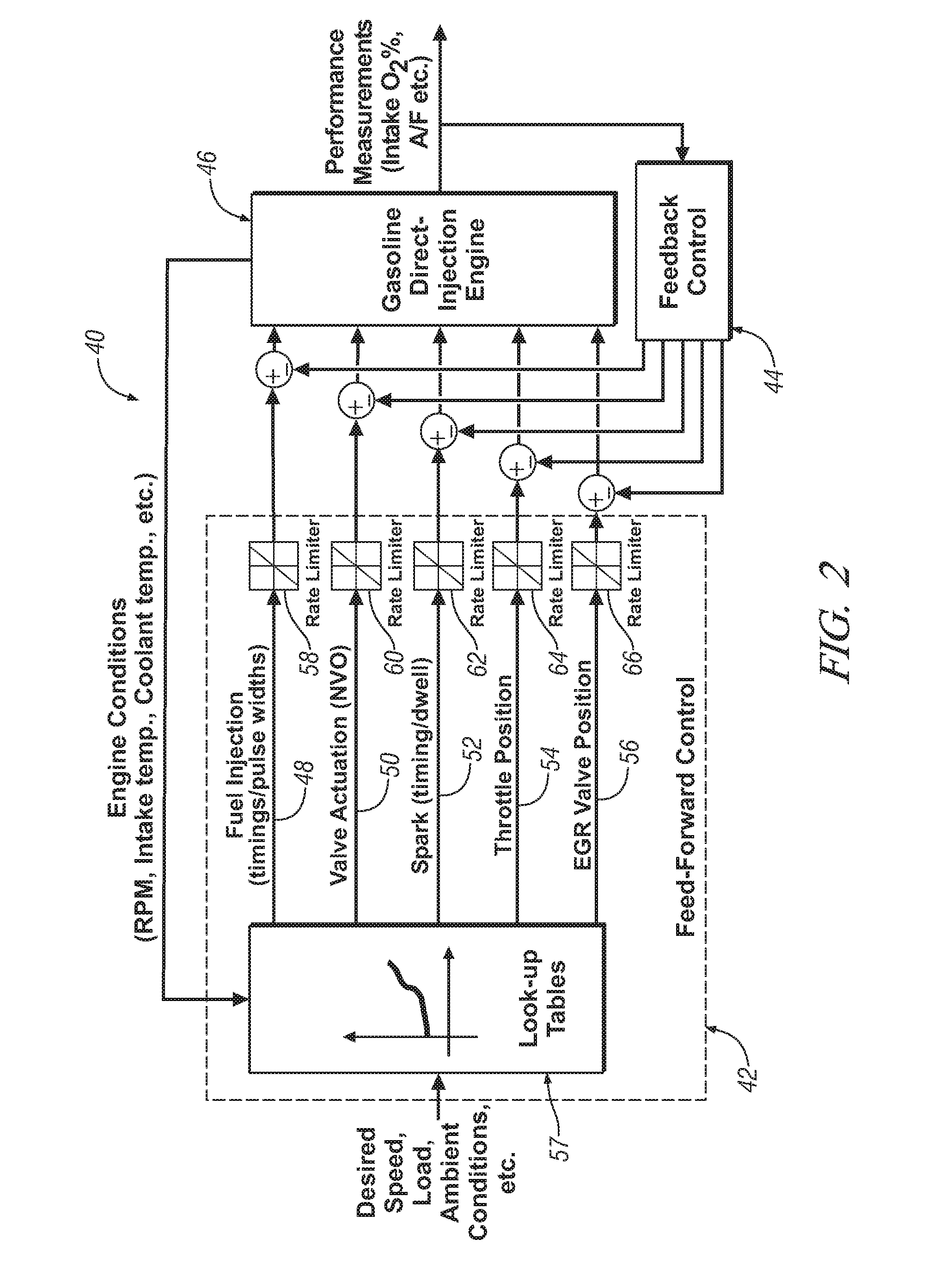
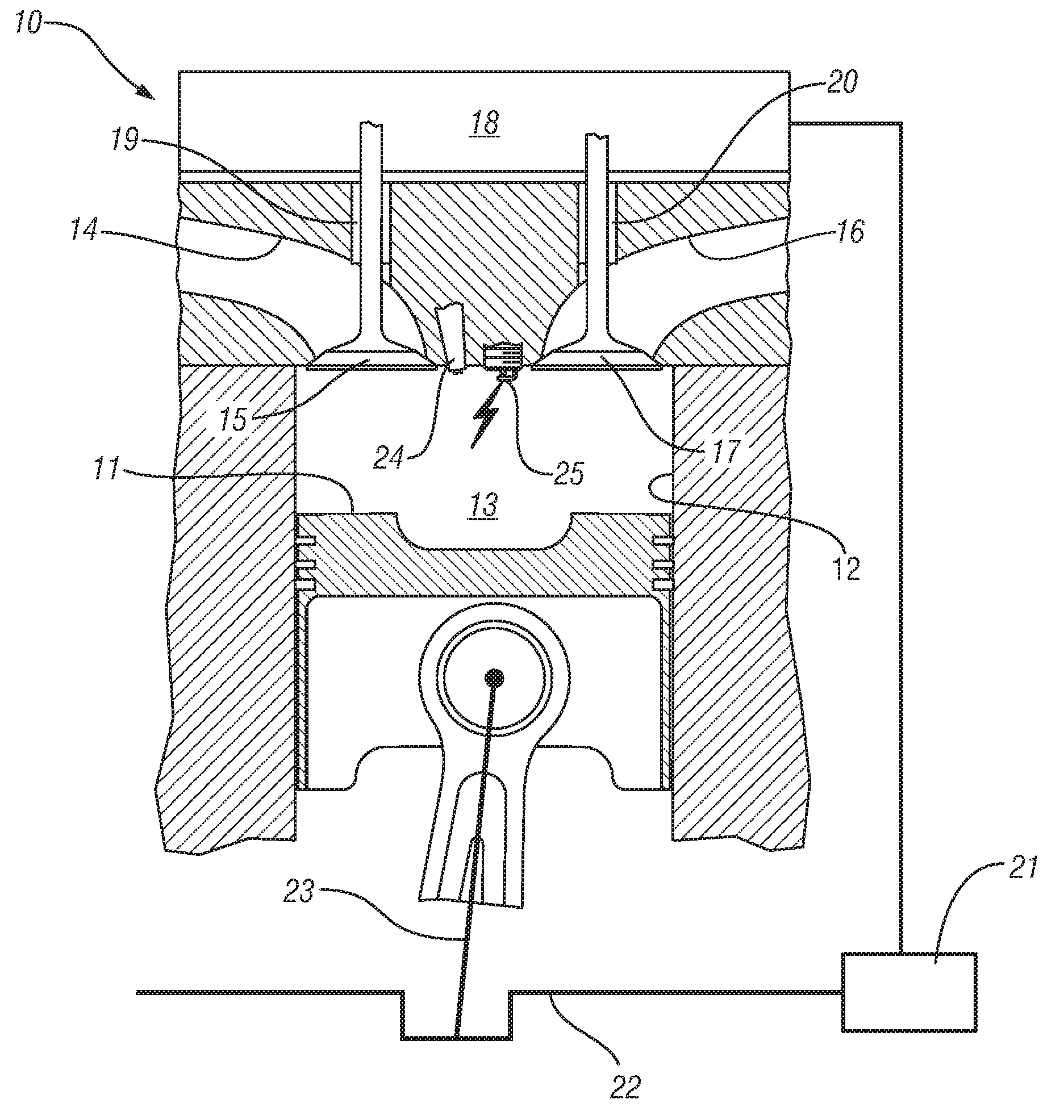
Homogeneous charge compression ignition engine operation
InactiveUS20070250256A1Robust operating set-pointsGuaranteed uptimeAnalogue computers for vehiclesElectrical controlData setEquilibrium constant
An HCCI engine is operated by controlling a plurality of engine operating parameters in accordance with a calibration data set representing equilibrium set-points of engine operation characterized by combustion phasing that is relatively least sensitive to cylinder charge temperature deviations.
Owner:GM GLOBAL TECH OPERATIONS LLC
Contact lens with a hydrophilic layer
ActiveUS9310627B2Maintain the health of the ophthalmic surface and wearer comfortMaintain sufficiencySpectales/gogglesOptical articlesRigid gas permeable lensHydrophilic polymers
Embodiments of the technology relate to a contact lens having a core that is coated by a hydrogel layer, and to methods of making such a lens. The coated lens can include a rigid gas permeable contact lens. The coated lens can also include a hybrid silicone and rigid gas permeable contact lens. In one aspect, embodiments provide for a coated contact lens comprising a lens core with a water equilibrium constant of less than about 2% comprising an outer surface; and a hydrogel layer covalently attached to at least a portion of the outer surface, the hydrogel layer adapted to contact an ophthalmic surface, wherein the hydrogel layer comprises a hydrophilic polymer population of one or more species.
Owner:TANGIBLE SCI LLC
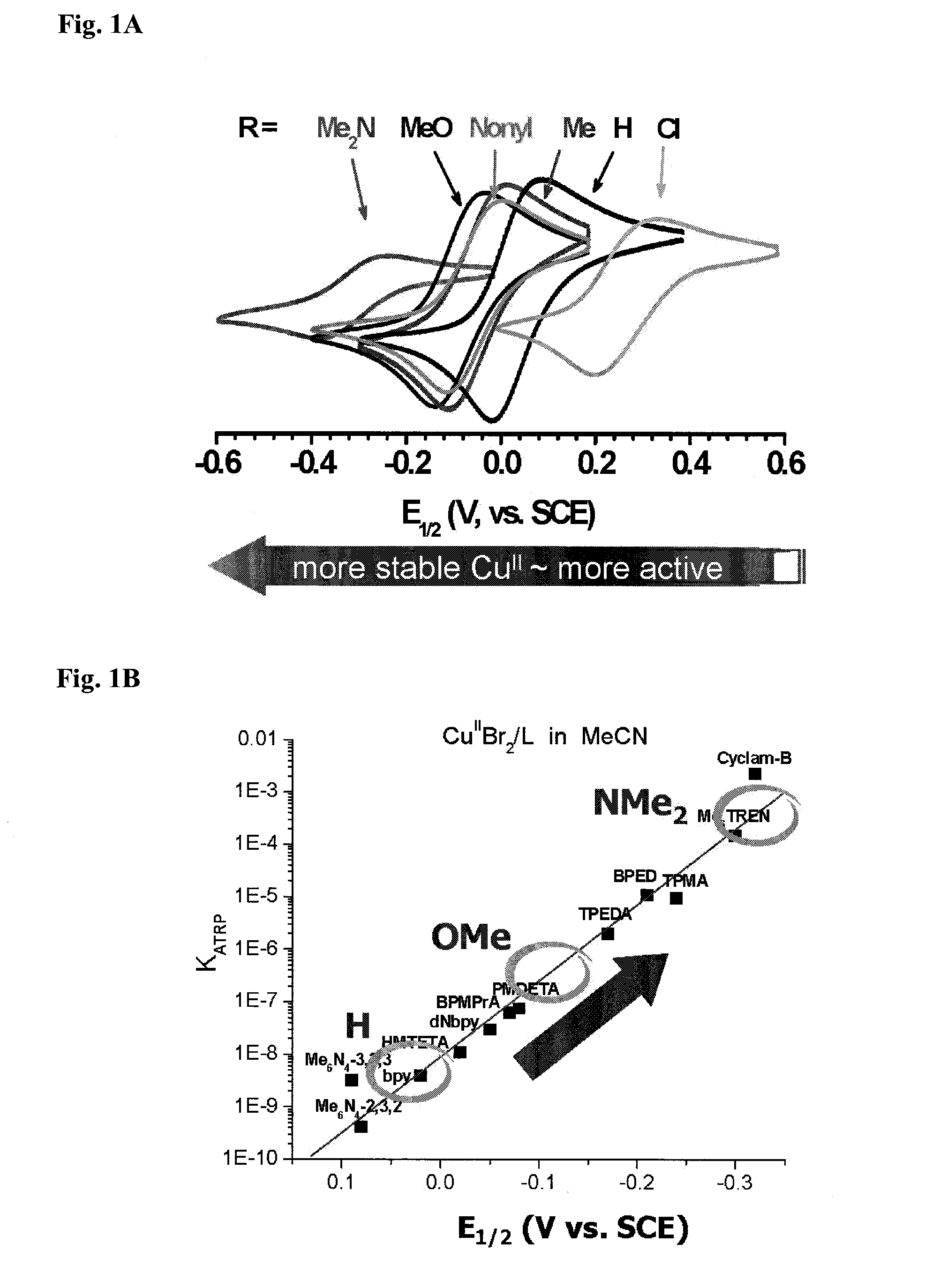
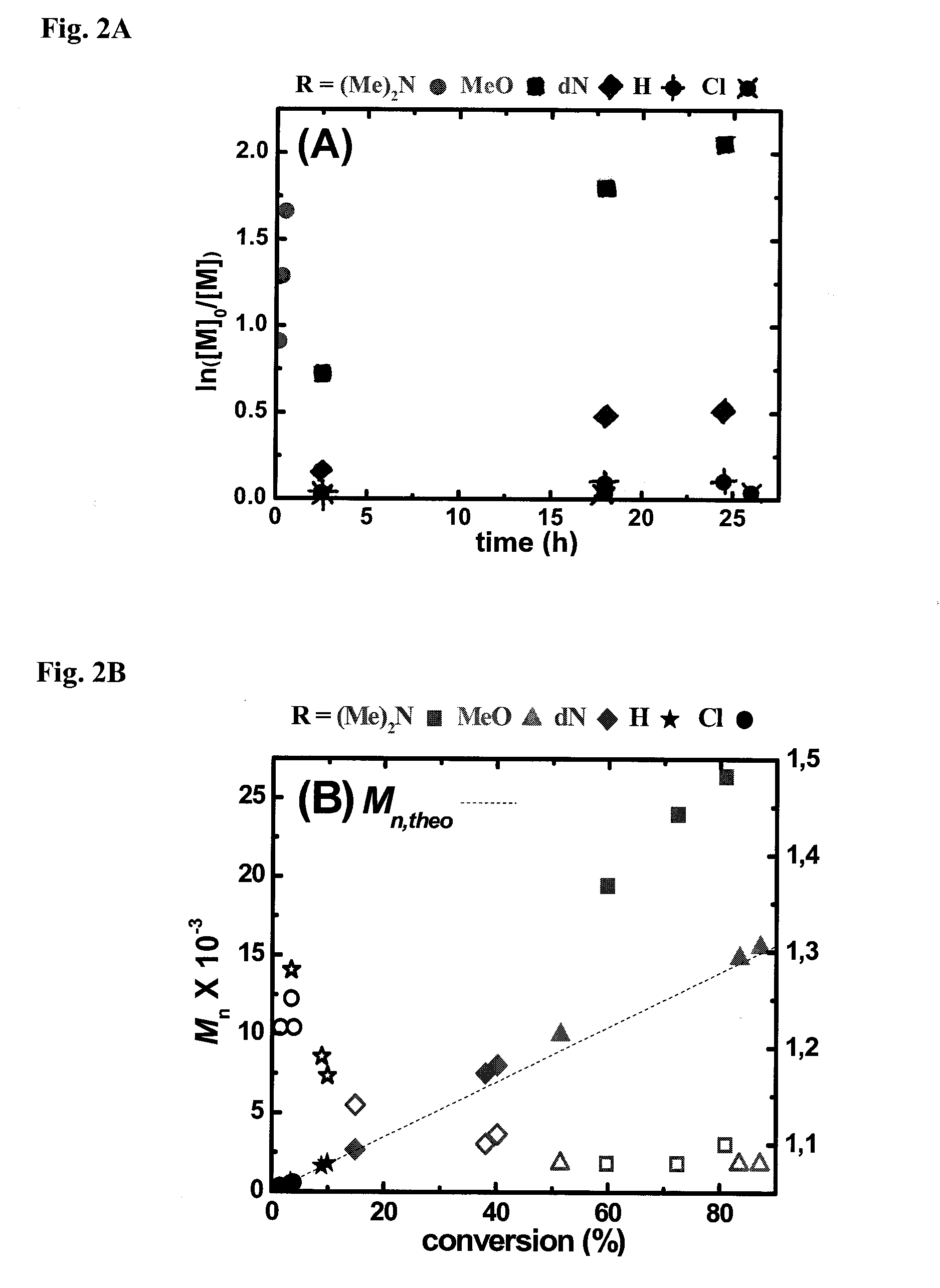
Ligands designed to provide highly active catalyst complexes
ActiveUS20150087795A1Organic-compounds/hydrides/coordination-complexes catalystsGroup 8/9/10/18 element organic compoundsEquilibrium constantUltimate tensile strength
A series of ligands with site specific electron donating substituents that form a catalyst complex with a transition metal and are suitable for catalysis of atom transfer radical reactions, including ATRP are described. Faster catalysis rates were observed allowing for low catalyst concentrations and linear increases in molecular weight with monomer conversion, and narrow molecular weight distributions. Cyclic voltammetry revealed that increasing the strength and number of conjugated electron donating groups resulted in more stable complexes and larger ATRP equilibrium constants.
Owner:CARNEGIE MELLON UNIV
Nonlinear spectroscopic methods for identifying and characterizing molecular interactions
ActiveUS7771938B2Remarkable effectSmall structureSugar derivativesPeptide/protein ingredientsNonlinear spectroscopyEquilibrium constant
Owner:WISCONSIN ALUMNI RES FOUND
Material mixing method of graphite negative electrode slurry
ActiveCN109904430ASmooth mixingReunion won't happenCell electrodesSecondary cellsGraphiteEquilibrium constant
The invention provides a material mixing method of graphite negative electrode slurry. The graphite negative electrode slurry comprises a first graphite material, a second graphite material and a third graphite material, wherein the average grain diameter of the first graphite material is d1, the average grain diameter of the second graphite material is d2, and the average grain diameter of the third graphite material is d3, wherein d1 is smaller than d2 which is smaller than d3, and d2 is equal to k*(d1+d3); k is equal to a*(c1+c3) / (c2+0.5), wherein a is an equilibrium constant, and a is greater than or equal to 1.1 and less than or equal to 1.3; c1, c2 and c3 represent percentages of the first graphite material, the second graphite material and the third graphite material; and c1+ c2 +c3is equal to 1, and c1+c2 is less than or equal to c3. The material mixing method comprises the following steps of carrying out mixing on the first graphite material, the second graphite material andthe third graphite material to obtain three slurry materials respectively, and then enabling the three slurry materials to be mixed according to a specific sequence to obtain the graphite negative electrode slurry with high dispersion degree. The graphite negative electrode slurry disclosed by the invention is high in graphite dispersion degree, free of agglomeration, free of sedimentation, high in storage stability, high in energy density and high in rate performance.
Owner:山东中信迪生电源有限公司
Method and computer system for quantum chemical modelling of molecules under non-equilibrium conditions
InactiveCN101019122ADesign optimisation/simulationSpecial data processing applicationsElectronic structureQuantum chemistry
Owner:ATOMISTIX
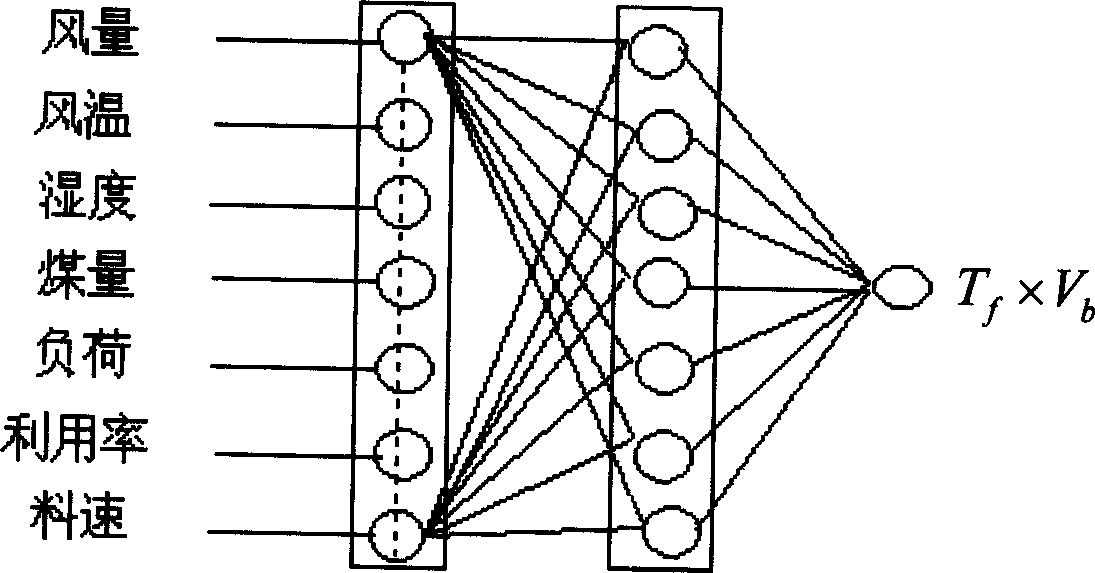
Method for stabilizing blast furnace operation based on equilibrium point
A method for stablilizing blast furnace operation based on balancing point is used in blast furnace ironmaking field. Blast furnace is controlled utilizing balance point in the invention. Firstly, choosing blast furnace spot data and denoting blast furnace balance point using multiplication of theoretical burning temperature bosh coal gas volume, then determining balance point influencing factor, using determined balance point influencing factor as input value, balance point as output value and choosing blast furnace spot data, learning rate being 0.30 from input layer to hidden layer, learning rate being 0.15 from hidden layer to output layer, momentum coefficient being 0.35, transformation function being tangent hyperbolic function , setting blast furnace balance point artificial nerval net prediction model, at last matching blast furnace valid data using nerval net exercise sample data, getting non-linear relationship of balance point with each blast furnace factor, controlling blast furnace according to non-linear relationship and blast furnace balance point value. Balance point data can guarantee the stable operation of blast furnace.
Owner:SHANGHAI JIAO TONG UNIV
Homogeneous charge compression ignition engine operation
InactiveUS7409285B2Guaranteed uptimeAnalogue computers for vehiclesElectrical controlData setEquilibrium constant
Owner:GM GLOBAL TECH OPERATIONS LLC
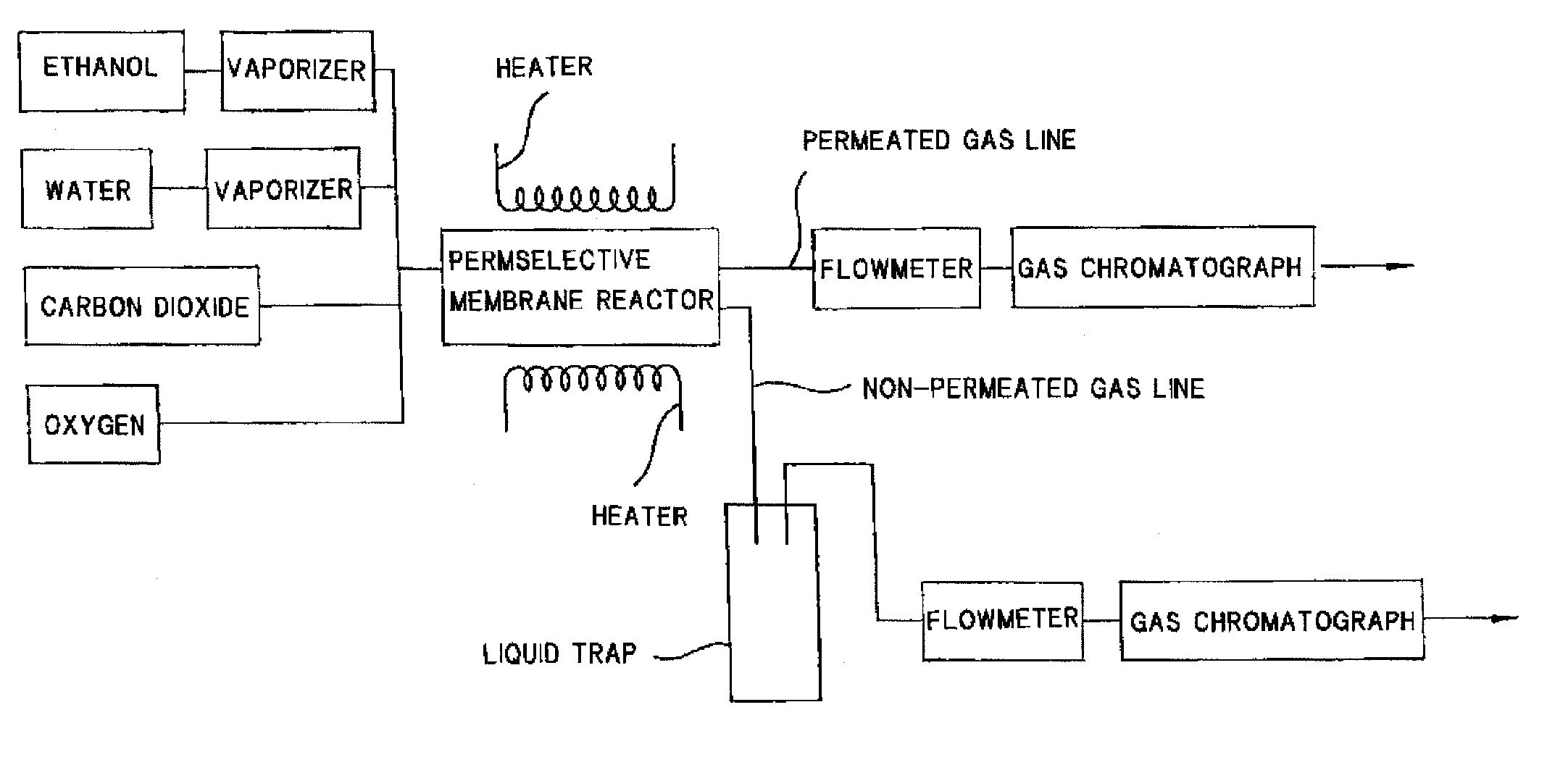
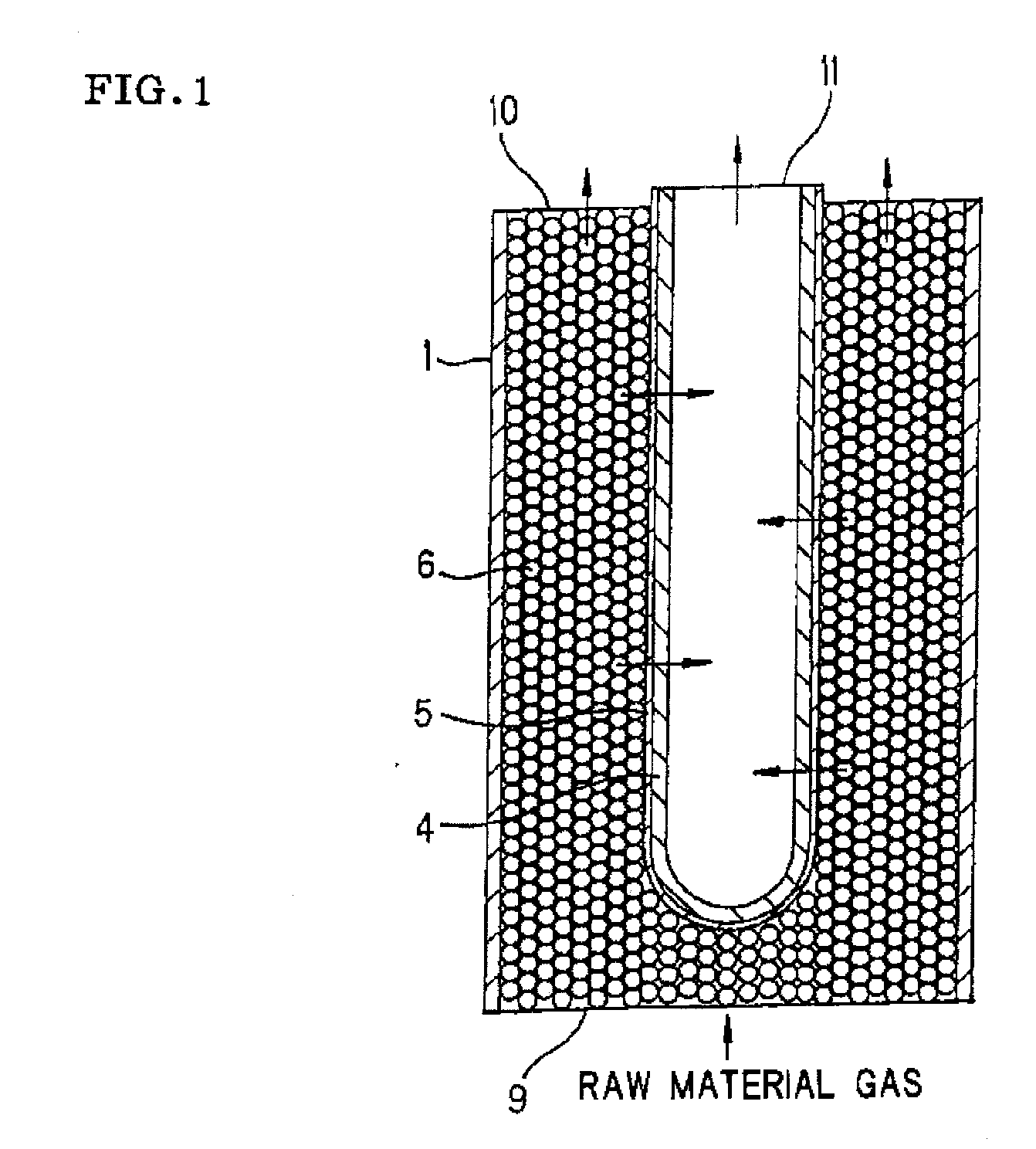
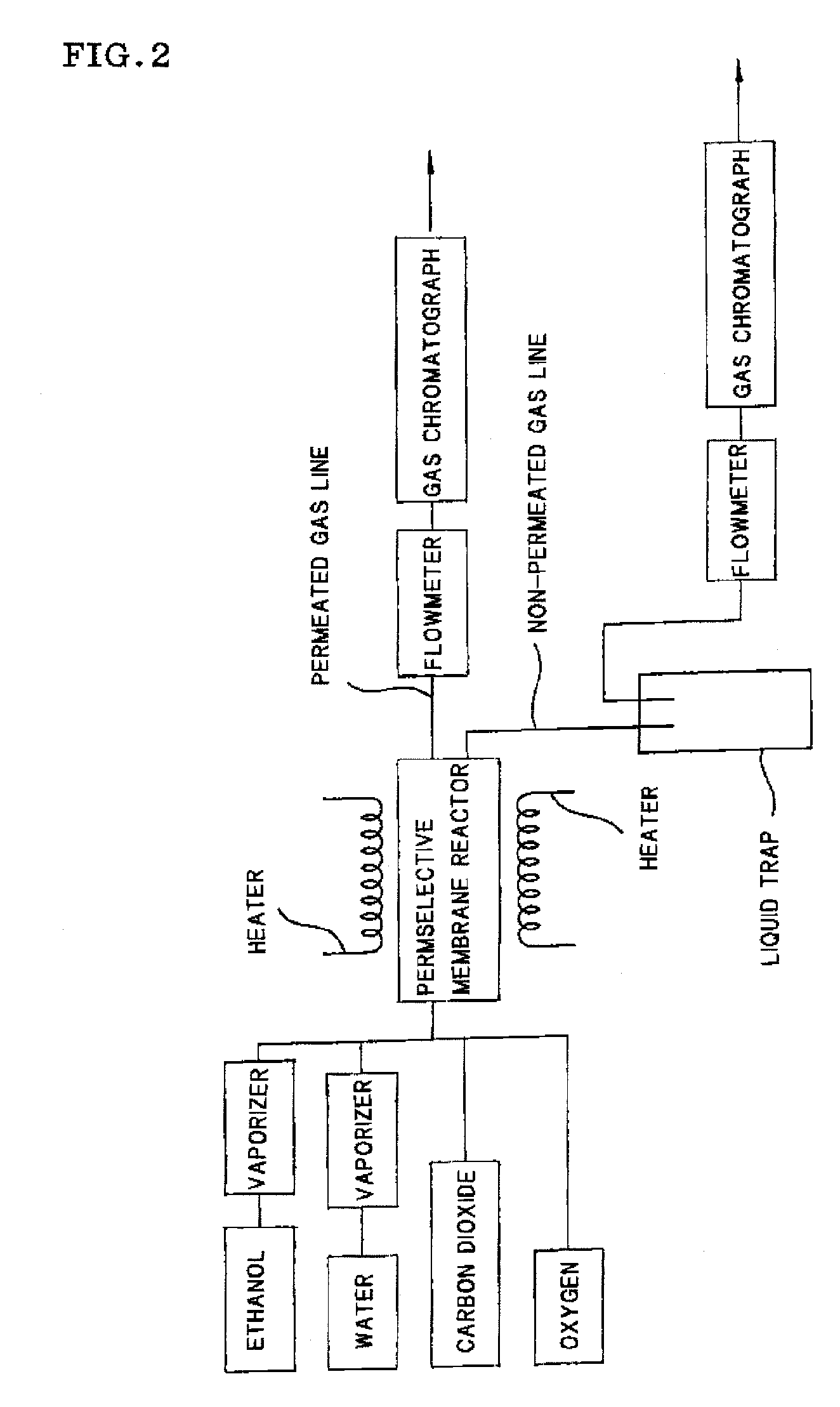
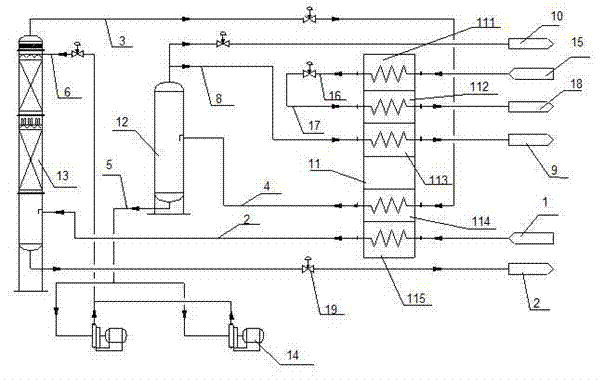
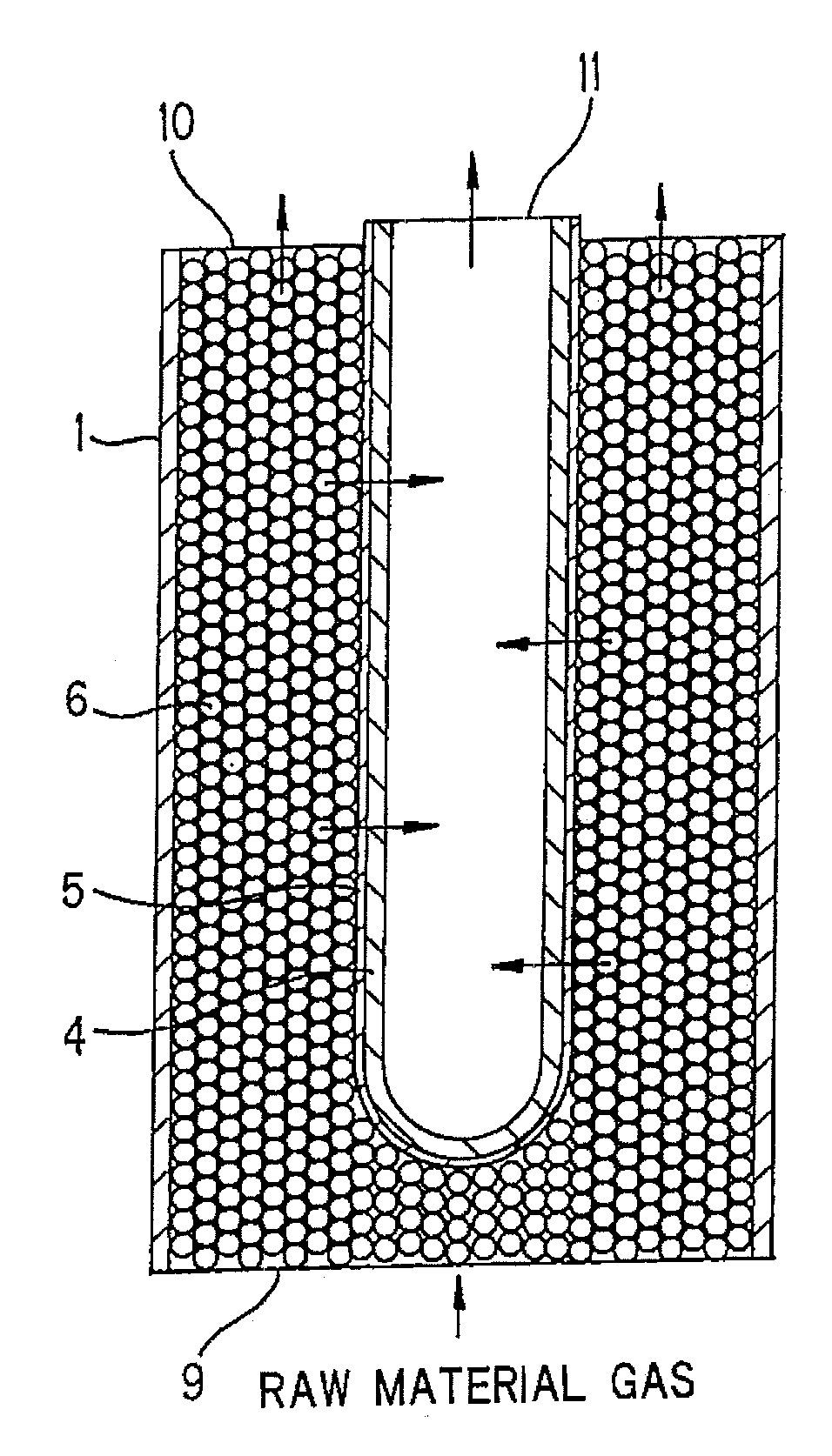
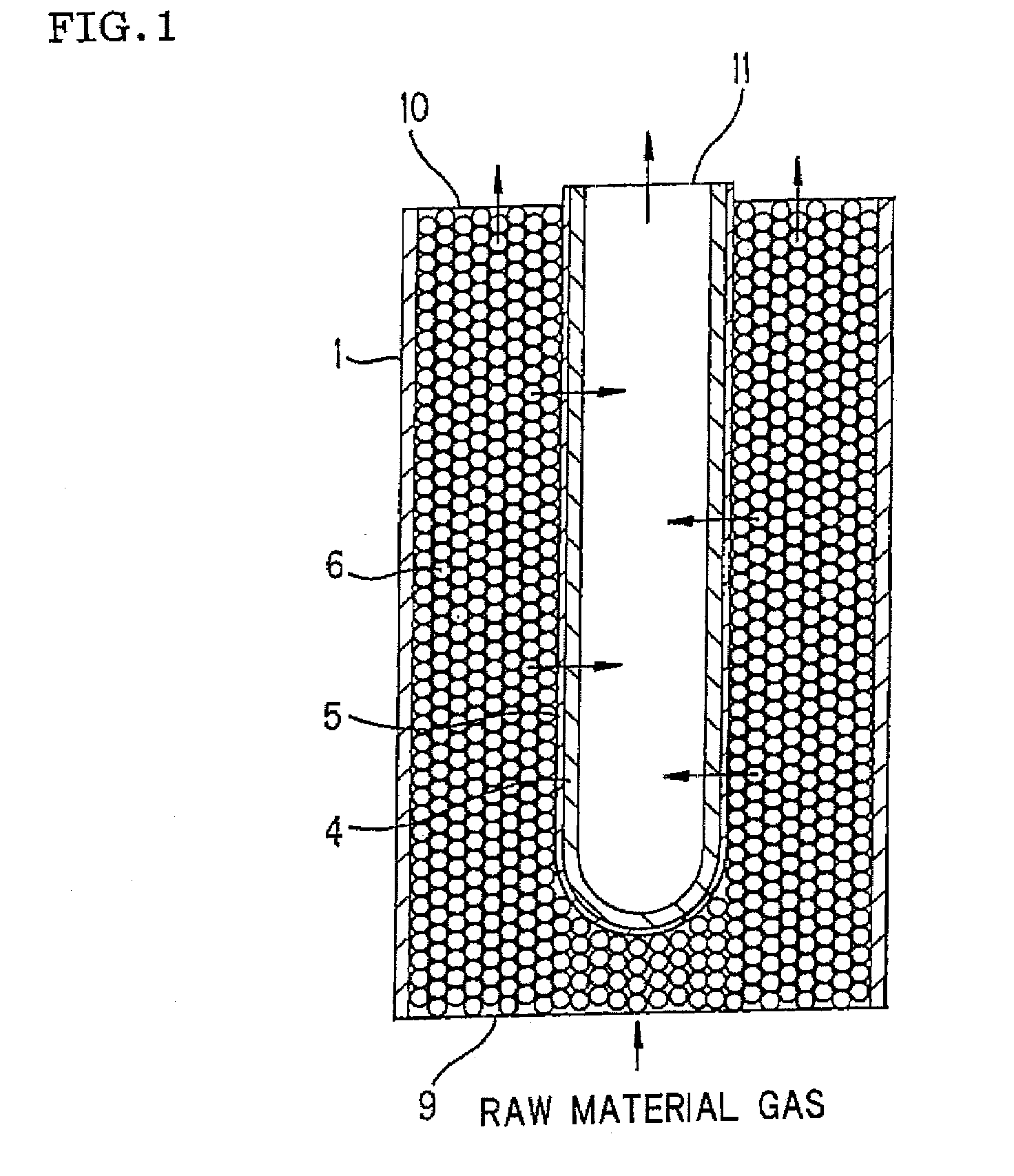
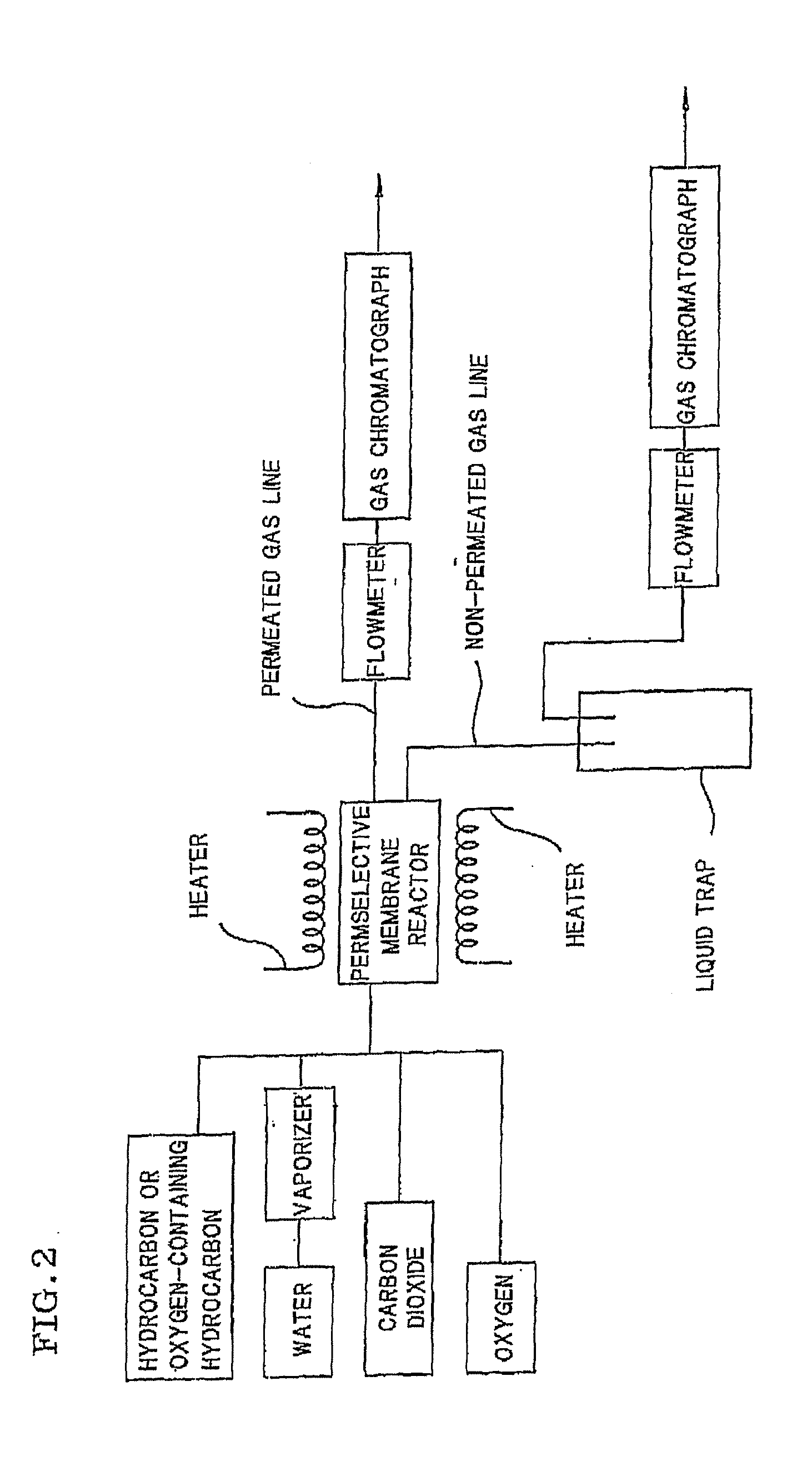
Process for producing hydrogen with permselective membrane reactor and permselective membrane reactor
ActiveUS20080107593A1Improve efficiencyReduce disproportionationCombination devicesGas treatmentHydrogenEquilibrium constant
In a process for producing hydrogen according to the present invention, hydrogen is produced under conditions where hydrogen recovery rate defined by the following equation is in the range of 60% to 99%: Hydrogen recovery rate=100×{A / (A+B)}wherein A denotes the amount of hydrogen that passes through the permselective membrane (the amount of permeated hydrogen) [ml / min], and B denotes the amount of hydrogen that does not pass through the permselective membrane (the amount of non-permeated hydrogen) [ml / min], and where α defined by the following equation is at least 0.6: α={(CO2) / (CO)2} / K wherein (CO2) denotes the partial pressure of carbon dioxide at the gas outlet of the reactor tube, (CO) denotes the partial pressure of carbon monoxide, and K denotes the equilibrium constant of the disproportionation reaction of carbon monoxide at the internal temperature of the reactor.
Owner:NGK INSULATORS LTD
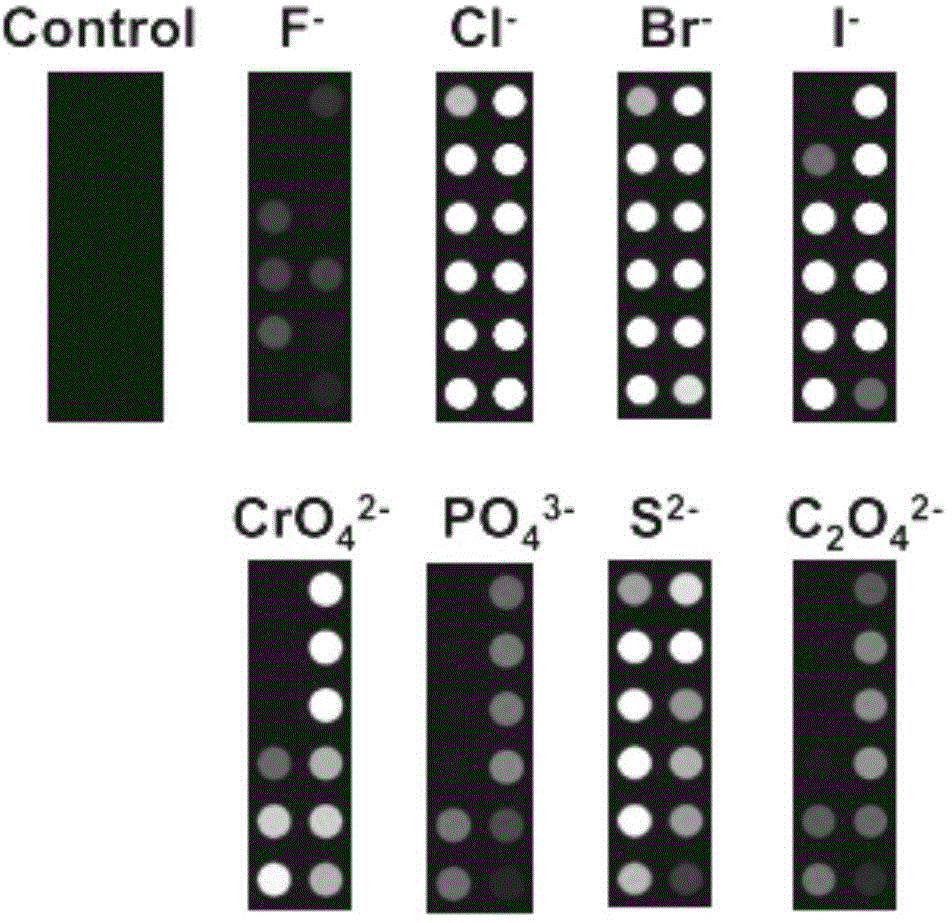
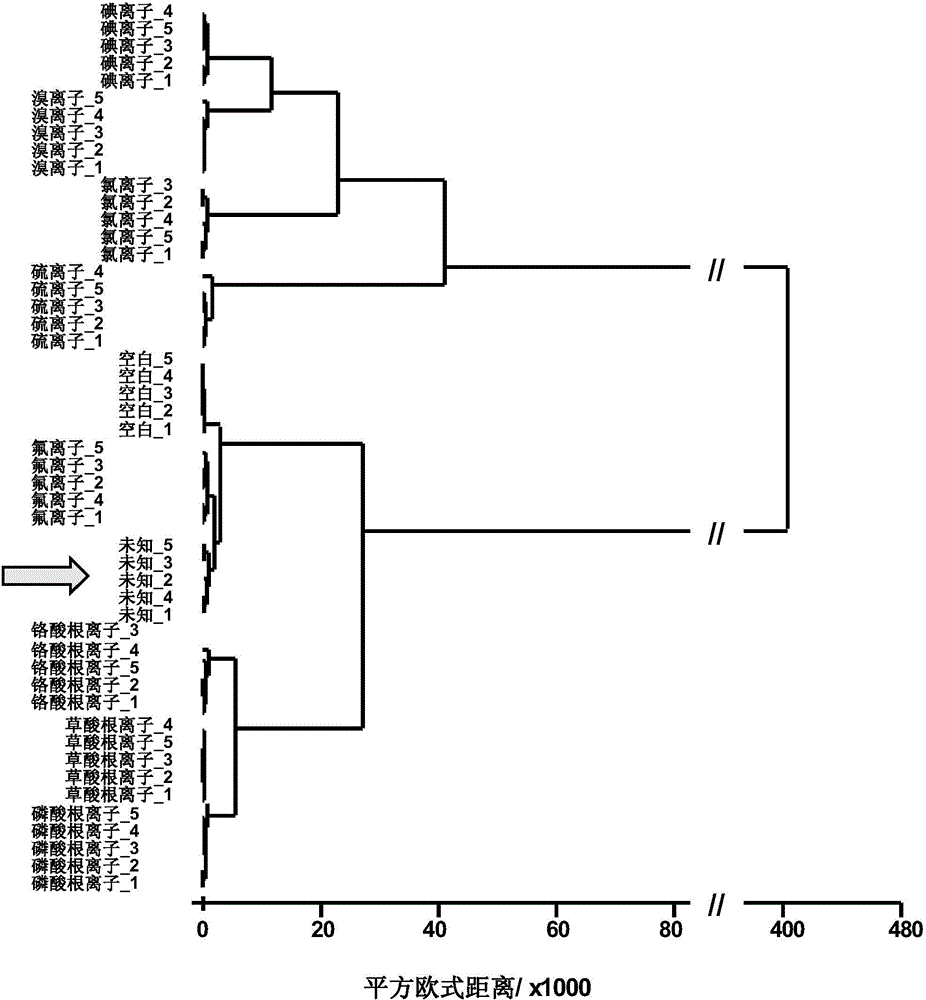
Indicator-array-based anion detection method
ActiveCN104931488AUniform and stable colorOvercome reflectionMaterial analysis by observing effect on chemical indicatorSensor arrayOrganic dye
The present invention relates to an indicator-array-based anion detection method used for the detection of multiple anions in water. Under the circumstance of certain concentration of an organic dye, chelates of different color gradients can be obtained by adding different amounts of same metal ion, and a sensor array is constructed by different concentrations of metal chelates; with the aid of the principle that anions in a water solution can be reacted with a metal to precipitate and even further reacted to produce a complex, metal ions can be replaced from the colored chelates, and the color changes. Due to different reaction equilibrium constants of different anions and a same indicator, the purpose of distinguishing of the anions can be achieved. By use of the method, whether S<2->, F<->, Cl<->, Br<->, I<->, CrO4<2->, PO4<3->, and C2O4<2-> anions in water exceed sewage or drinking water national discharge standards can be quickly determined, and by use of cluster analysis, principal component analysis and other mathematical statistic methods, unknown anions can be qualitatively or semi-quantitatively analyzed.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
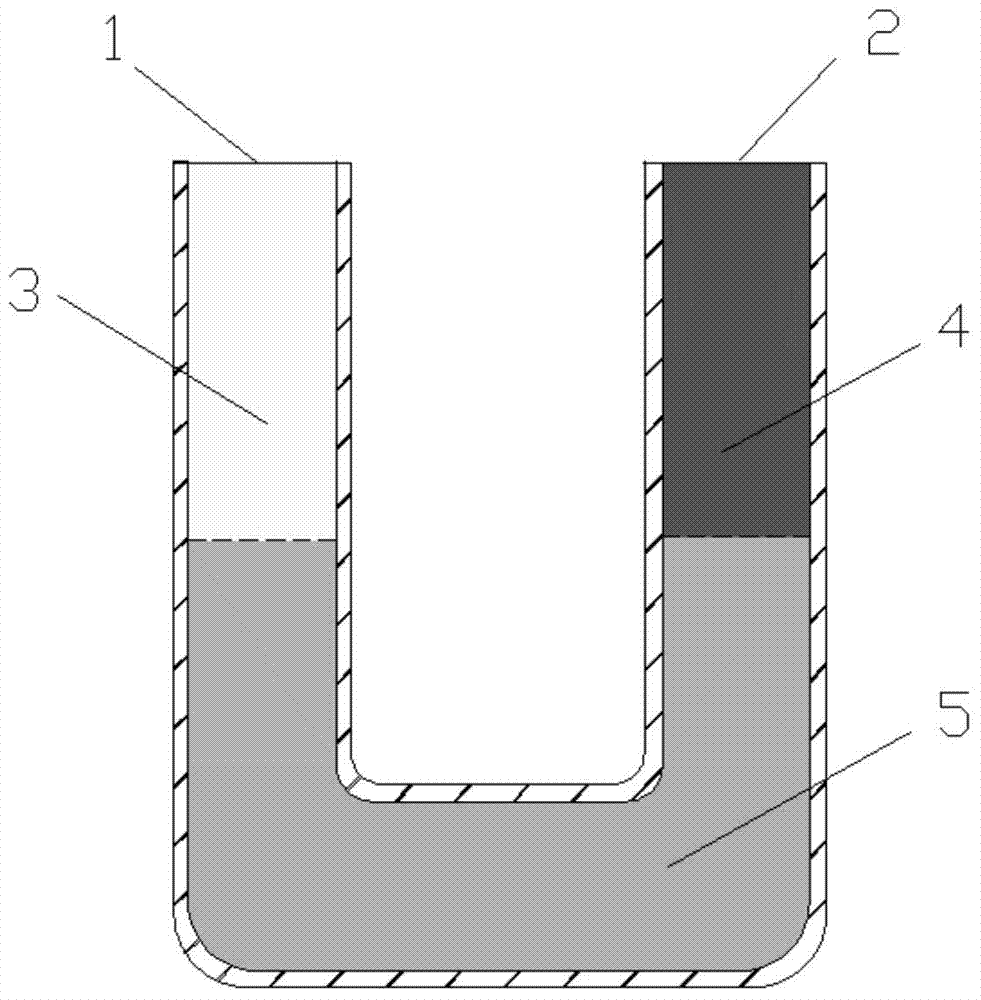
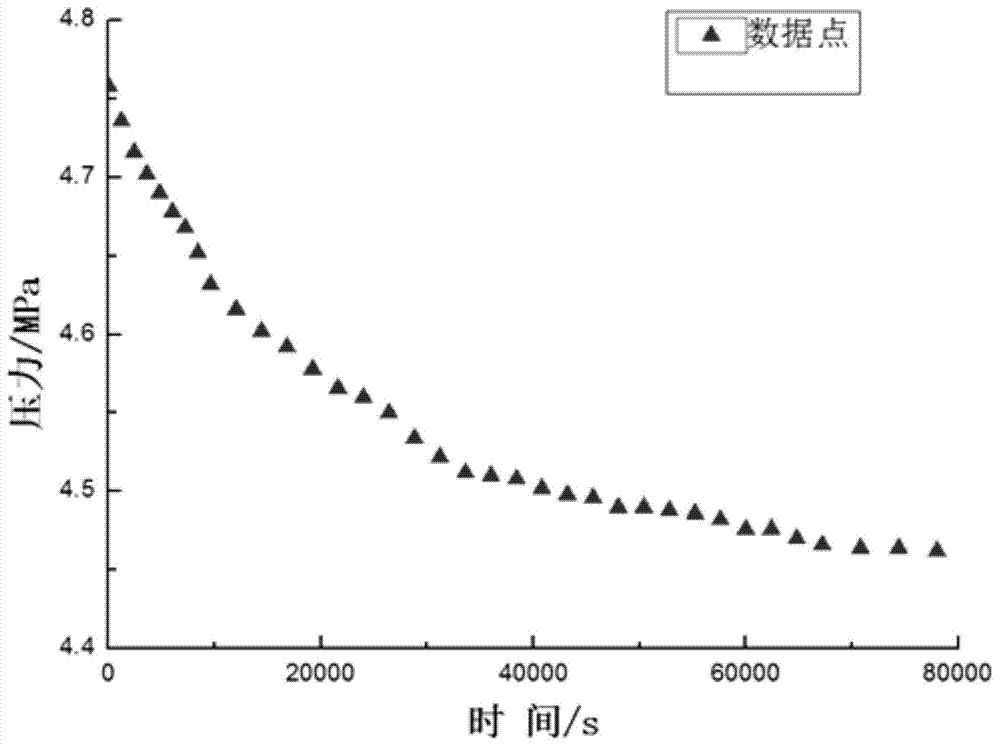
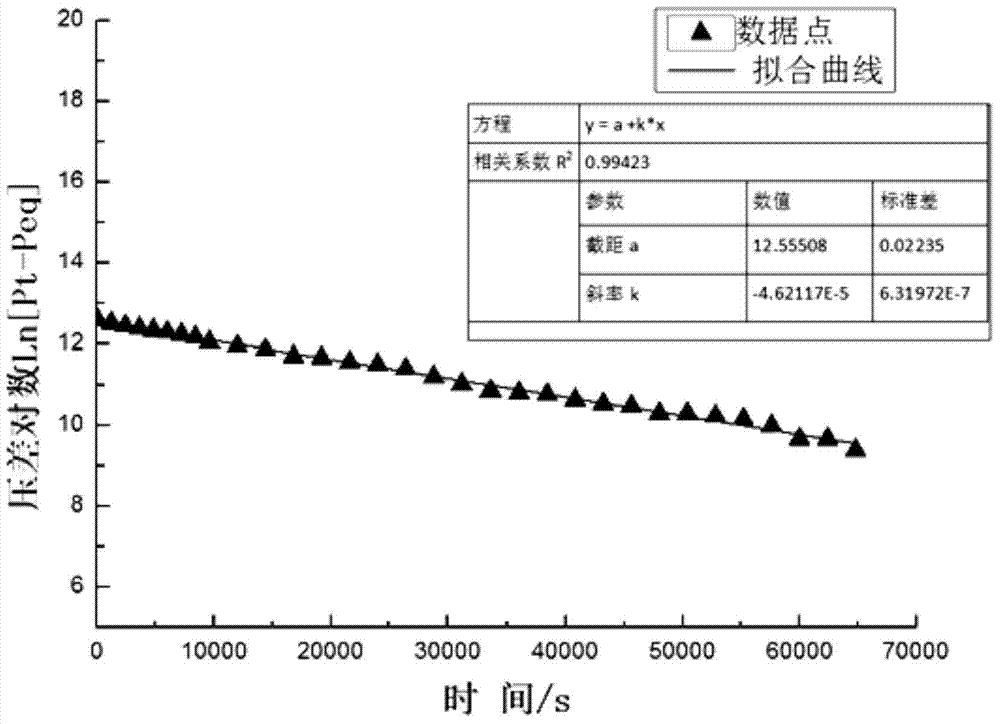
Method for measuring diffusion coefficient and equilibrium concentration of CO2 in process of diffusion from water phase to oil phase
ActiveCN104502236AEasy to operateClear principleSurface/boundary effectSpecial data processing applicationsDiffusionChemical physics
The invention relates to a method for measuring the diffusion coefficient and the equilibrium concentration of CO2 in the process of diffusion from a water phase to an oil phase. According to the method, the water-phase saturated CO2 at the bottom of a U-shaped pipe is formed into saturated carbonated water; CO2 is injected into the end a of the U-shaped pipe, and crude oil is injected into the end b of the U-shaped pipe; as CO2 in the water phase diffuses to the oil phase, CO2 in the saturated carbonated water is not saturated again, and therefore, the gaseous-phase CO2 is dissolved in the carbonated water. Pressure change due to the diffusion of the CO2 to the carbonated water is measured, and then the diffusion coefficient of the CO2 during diffusion from the water phase to the oil phase and the equilibrium concentration of the CO2 in the crude oil after equilibrium can be obtained in combination with a pressure drop formula. According to the method, the method for measuring the diffusion coefficient of the CO2 in the process of diffusion from the water phase to the oil phase by use of an improved PVT pressure drop method is provided for the first time, and the method is simple to operate and clear in principle; the diffusion coefficient can be obtained without directly measuring the concentration change of CO2 in the process of diffusion from the water phase to the oil phase; the method is significant for studying the migration and distribution of CO2 between the water phase and the oil phase and guiding oil-gas field development.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
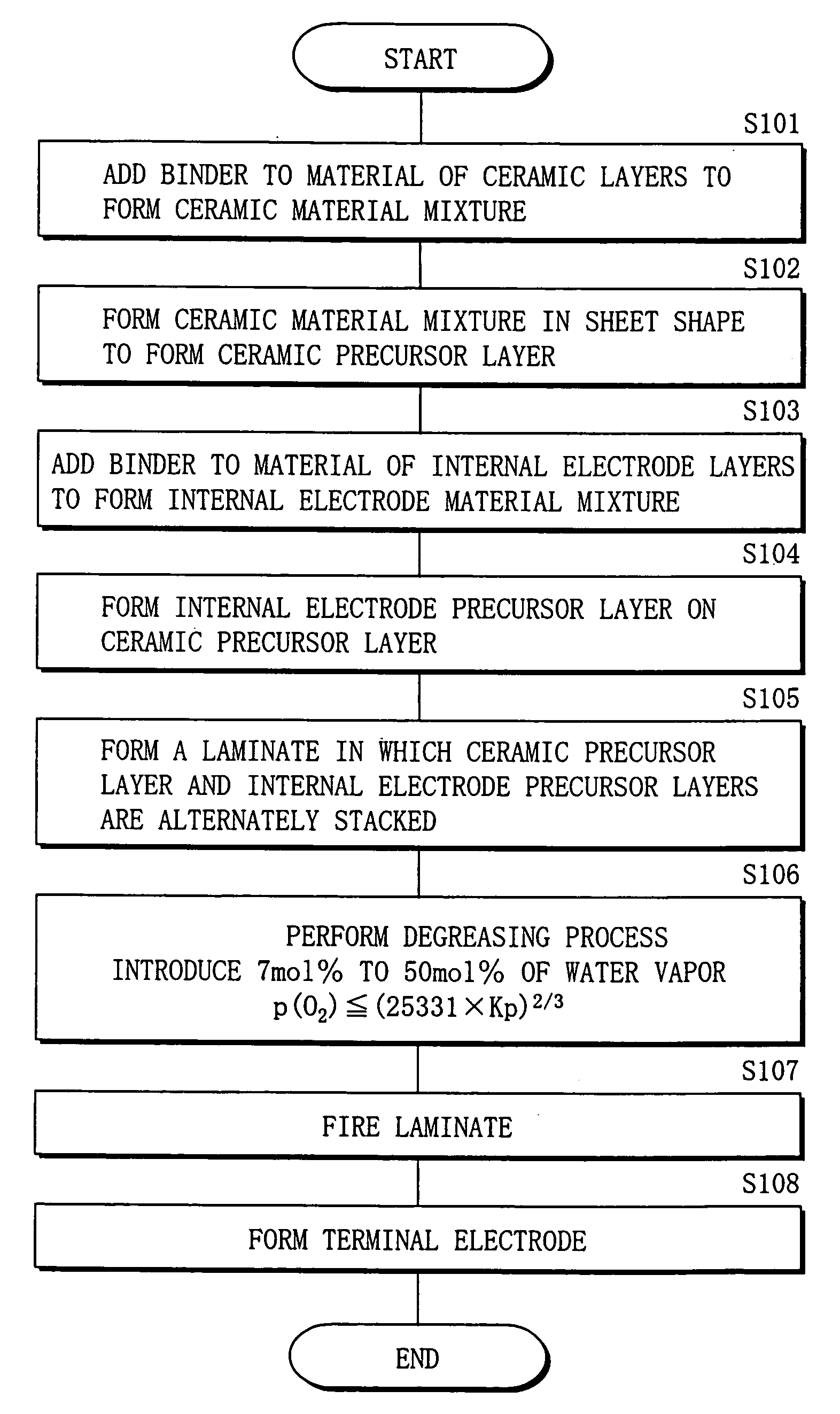
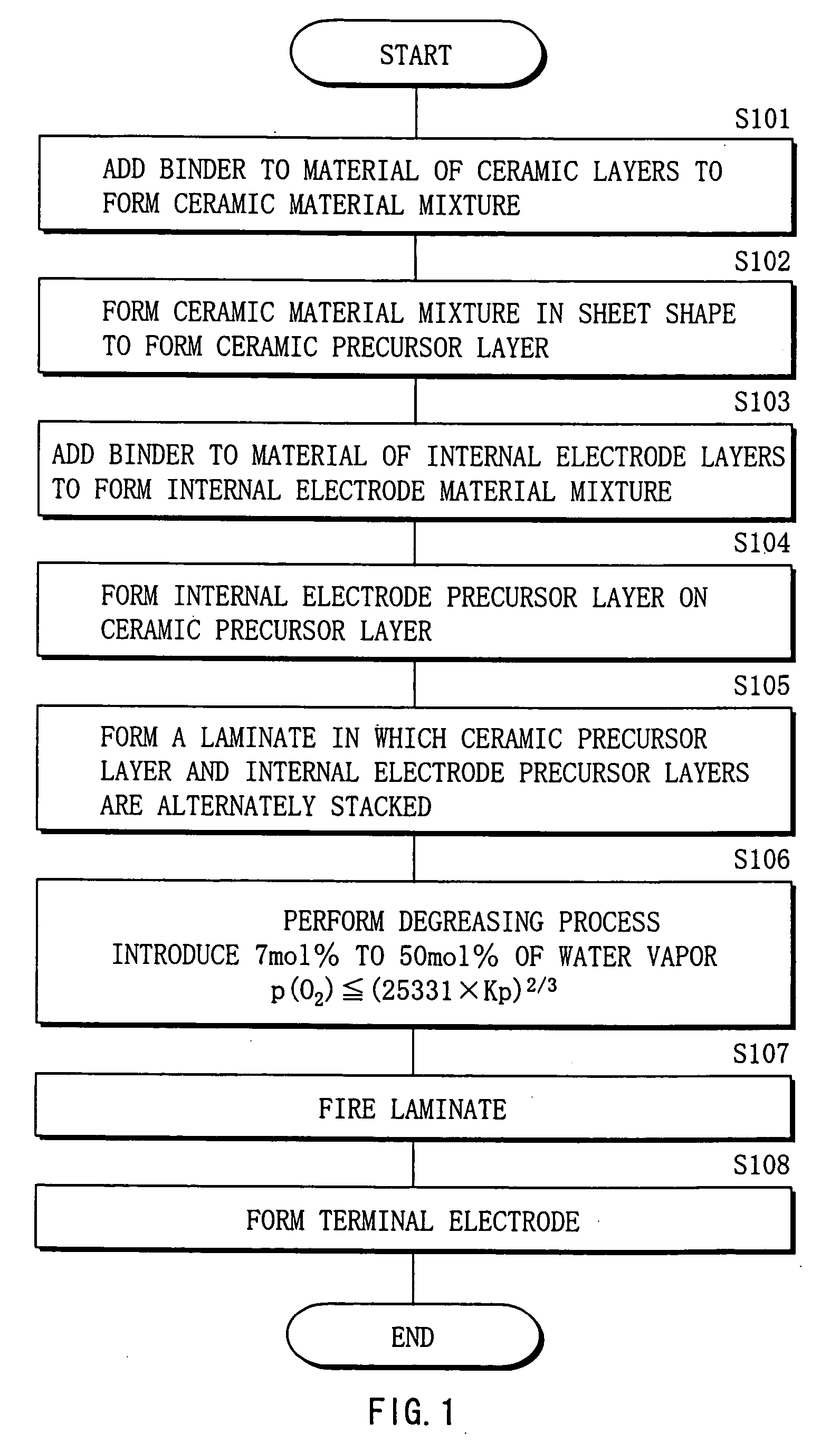
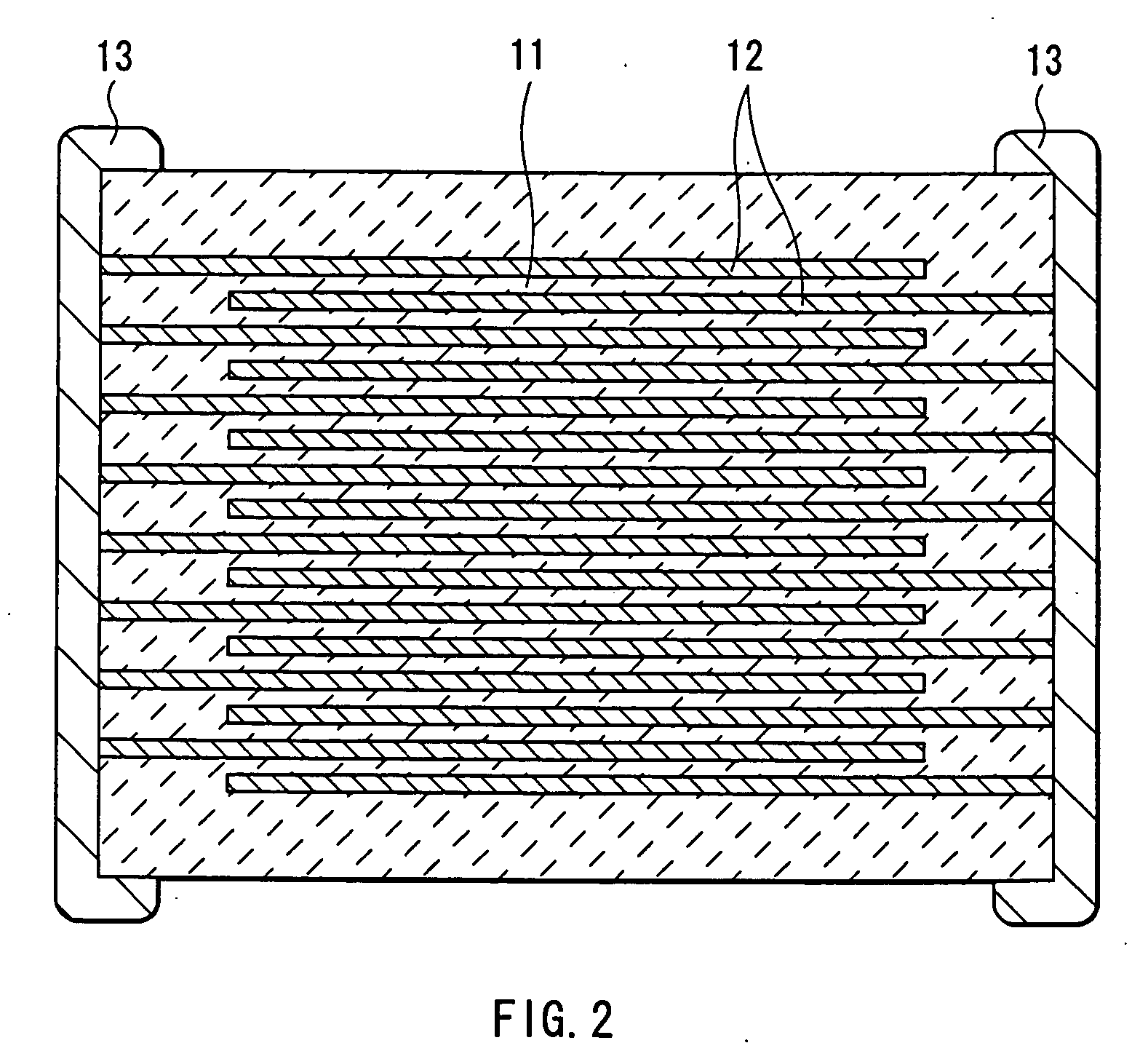
Method of manufacturing multilayer ceramic device
InactiveUS20050213283A1Maintain good propertiesAvoid layeringPiezoelectric/electrostrictive device manufacture/assemblyFixed capacitor dielectricHydrogenWater vapor
A method of manufacturing a multilayer ceramic device capable of obtaining a superior multilayer ceramic device even if the number of layers increases or even if the multilayer ceramic device is upsized, and specifically suitable for manufacturing a multilayer piezoelectric device is provided. After a laminate in which ceramic precursor layers including a raw material of a ceramic layer and internal electrode precursor layers including copper metal as a raw material of an internal electrode layer are alternately stacked is formed, the laminate is heated to degrease the laminate. At this time, an atmospheric gas including an inert gas, 7 mol % to 50 mol % of water vapor, and, if necessary, hydrogen is used to adjust an oxygen partial pressure within a range of p(O2)≦(25331×Kp)2 / 3, where p(O2) represents an oxygen partial pressure; Kp represents a water dissociation equilibrium constant; and the pressure unit is Pa. Thereby, while the oxidation of copper metal can be prevented, a binder can be sufficiently decomposed and removed, and residual carbon can be reduced.
Owner:TDK CORPARATION
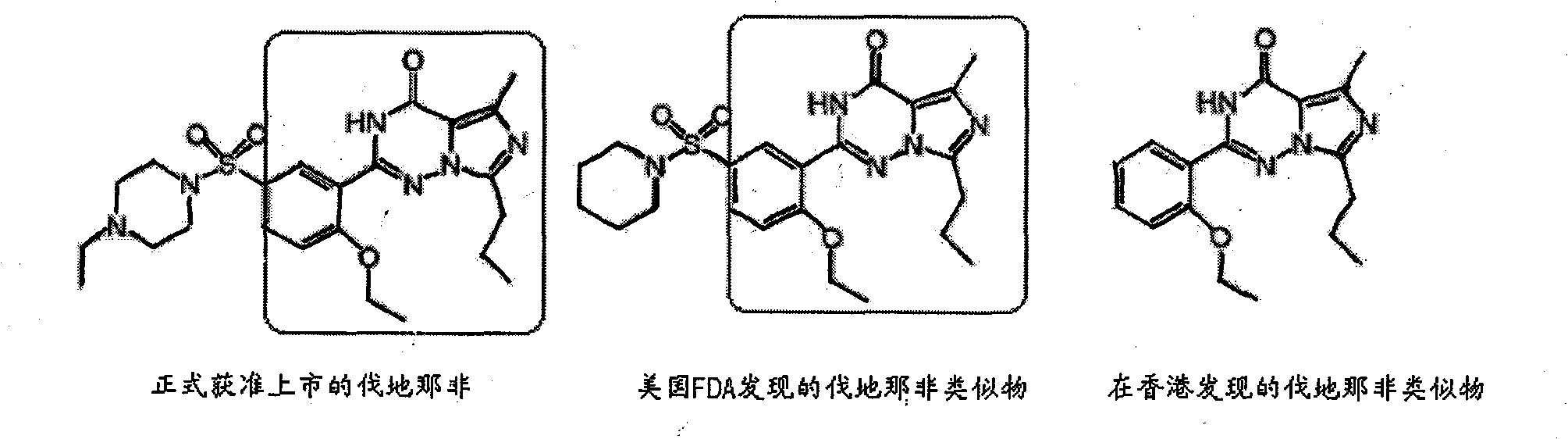
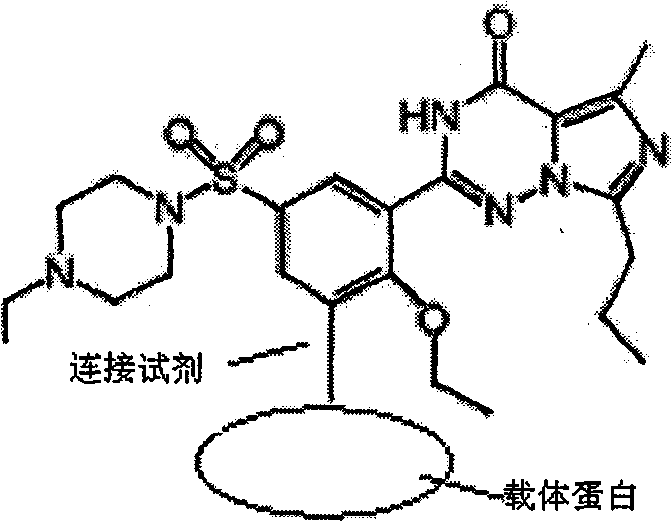
Preparation method for monoclonal antibody specifically binding vardenafil and analog thereof
The invention relates to a preparation method for a monoclonal antibody specifically binding vardenafil and an analog thereof. The preparation method comprises the steps of: synthesizing artificial immunizing antigens and artificial testing antigens, screening mice under immune state and the monoclonal antibody, and conducting structural specificity identification of the monoclonal antibody, specifically, mixing M-BSA with the vardenafil, regulating pH, adding a glutaraldehyde coupling agent, slowly stirring with a magnetic stirrer for reaction, dialyzing with distilled water, freezing and drying under vacuum condition, and synthesizing the artificial immunizing antigens; mixing M-OVA with the vardenafil, regulating pH, adding a glutaraldehyde coupling agent, slowly stirring with a magnetic stirrer for reaction, dialyzing with distilled water, freezing and drying under vacuum condition, and synthesizing the artificial testing antigens; and using a competitive inhibition ELISA method to detect the coasensual reaction between the monoclonal antibody and the vardenafil and piperidenafil and identifying the monoclonal antibody which has high coasensual reaction rate and can specifically bind the vardenafil and the analog thereof. The affinity equilibrium constant of the monoclonal antibody prepared by the method on the vardenafil and the analog thereof is higher than 10M.
Owner:NANCHANG UNIV
Refined heavy-hydrocarbon removal system for recovering LNG/LPG/NGL from petroleum associated gas
ActiveCN104726127AReduced heavy hydrocarbon contentHigh precisionLiquid hydrocarbon mixture recoveryGaseous fuelsEngineeringEquilibrium constant
The invention provides a refined heavy-hydrocarbon removal system for recovering LNG / LPG / NGL from petroleum associated gas, which comprises a raw material associated gas inlet pipeline, an LPG cold box, a low-temperature separator and a refined removed heavy hydrocarbon absorption tower, wherein an outlet of a lower box of the LPG cold box is connected with the refined removed heavy hydrocarbon absorption tower by a pipeline, an outlet at the bottom of the refined removed heavy hydrocarbon absorption tower is connected with a tower-bottom liquid phase pipeline, an outlet at the top of the refined removed heavy hydrocarbon absorption tower is connected with a lower middle box of the LPG cold box by a pipeline, the lower middle box is connected with the low-temperature separator by a pipeline, the bottom of the low-temperature separator is connected with an absorbent booster pump by a pipeline, and the absorbent booster pump is connected with the refined removed heavy hydrocarbon absorption tower by a pipeline. By using the characteristic that the equilibrium constants of hydrocarbon substances at different temperatures and consisting of different components are different, the system dilutes the heavy hydrocarbon content of associated gas, thereby achieving the purpose of reducing the heavy hydrocarbon content of dry gas; the temperature of associated gas is reduced two times by using the cold box so as to meet different temperature requirements of absorption and separation; and due to the adoption of the absorption tower, the precision of heavy hydrocarbon removal is improved.
Owner:XIAN CHANGQING TECH ENG
Process for producing hydrogen with permselective membrane reactor and permselective membrane reactor
InactiveUS20080241058A1Efficient separationIncreasing the thicknessMembranesProductsHydrogenEquilibrium constant
A method for producing hydrogen including the steps of supplying a raw material gas from a gas inlet of a reactor tube; producing a gas mixture containing hydrogen, carbon monoxide, and carbon dioxide by a reforming reaction and a shift reaction; recovering, from a discharge outlet of a separator tube, hydrogen being isolated by passing through a permselective membrane into the separator tube from the gas mixture; and discharging other gas components incapable of passing through the permselective membrane from a gas outlet of the reactor. Hydrogen is produced under conditions where α defined by the following equation is in the range of 0.4 to 100:α={(CO2) / (CO)2} / Kwherein (CO2) and (CO) denote the partial pressures of carbon dioxide and carbon monoxide at the gas outlet and K denotes the equilibrium constant of the disproportionation reaction of carbon monoxide at the internal temperature of the reactor tube.
Owner:NGK INSULATORS LTD
Purification of substances by reaction affinity chromatography
InactiveUS7094351B2Ion-exchange process apparatusOther chemical processesStationary phaseEquilibrium constant
The invention relates to the separation of at least one target from a sample composition using a naturally reversible reaction comprising the formation of at least one covalent bond. One embodiment of the invention is a chromatographic method in which the affinity of a stationary phase for at least one target is based on the equilibrium constant of such a naturally reversible reaction between the stationary phase and the target.
Owner:CORCORAN ROBERT C
Process for removing fluorosurfactant from aqueous fluoropolymer dispersions and reducing scum formation
InactiveUS20060135680A1Reducing fluorosurfactant contentPrevent scumFibre treatmentFluoropolymerEquilibrium constant
A process for reducing fluorosurfactant content of a stabilized fluorosurfactant-containing aqueous fluoropolymer dispersion containing ferric ions by contacting the fluoropolymer dispersion with strong base anion exchange resin to reduce fluorosurfactant content to a predetermined level, the anion exchange resin being in the hydroxide form, separating the dispersion from the anion exchange resin, and adding an effective amount of chelating agent having an equilibrium constant when complexed with iron of at least 1018 prior to the contacting with the ion exchange resin for complexing ferric ions to prevent scum formation.
Owner:THE CHEMOURS CO FC LLC
Process for removing fluorosurfactant from aqueous fluoropolymer dispersions and reducing scum formation
Owner:THE CHEMOURS CO FC LLC
Optically rebalanced accelerometer
InactiveUS6867411B2Reduce weightExcess buildupPhotometry using reference valueAcceleration measurement using interia forcesOptical radiationLocation detection
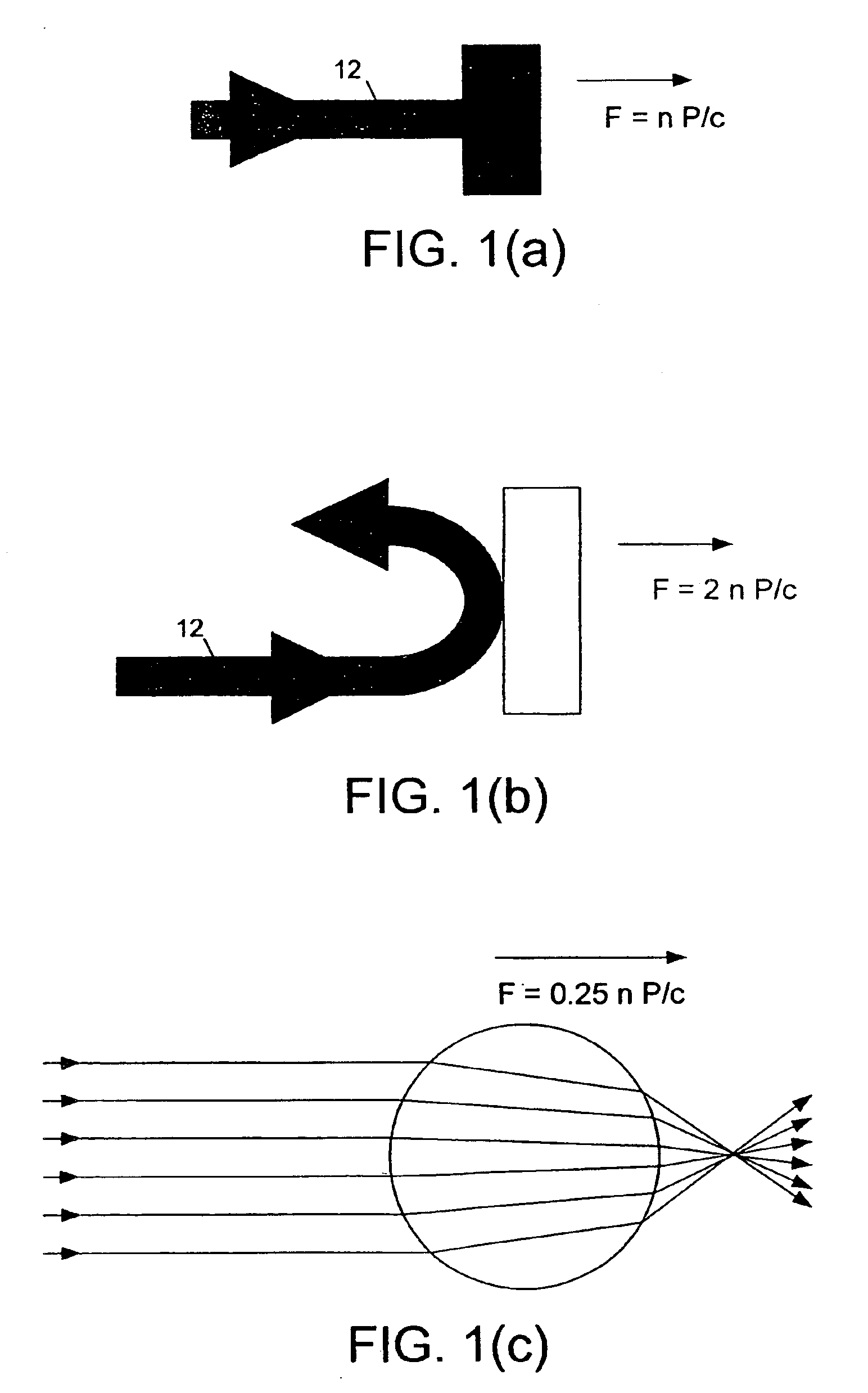
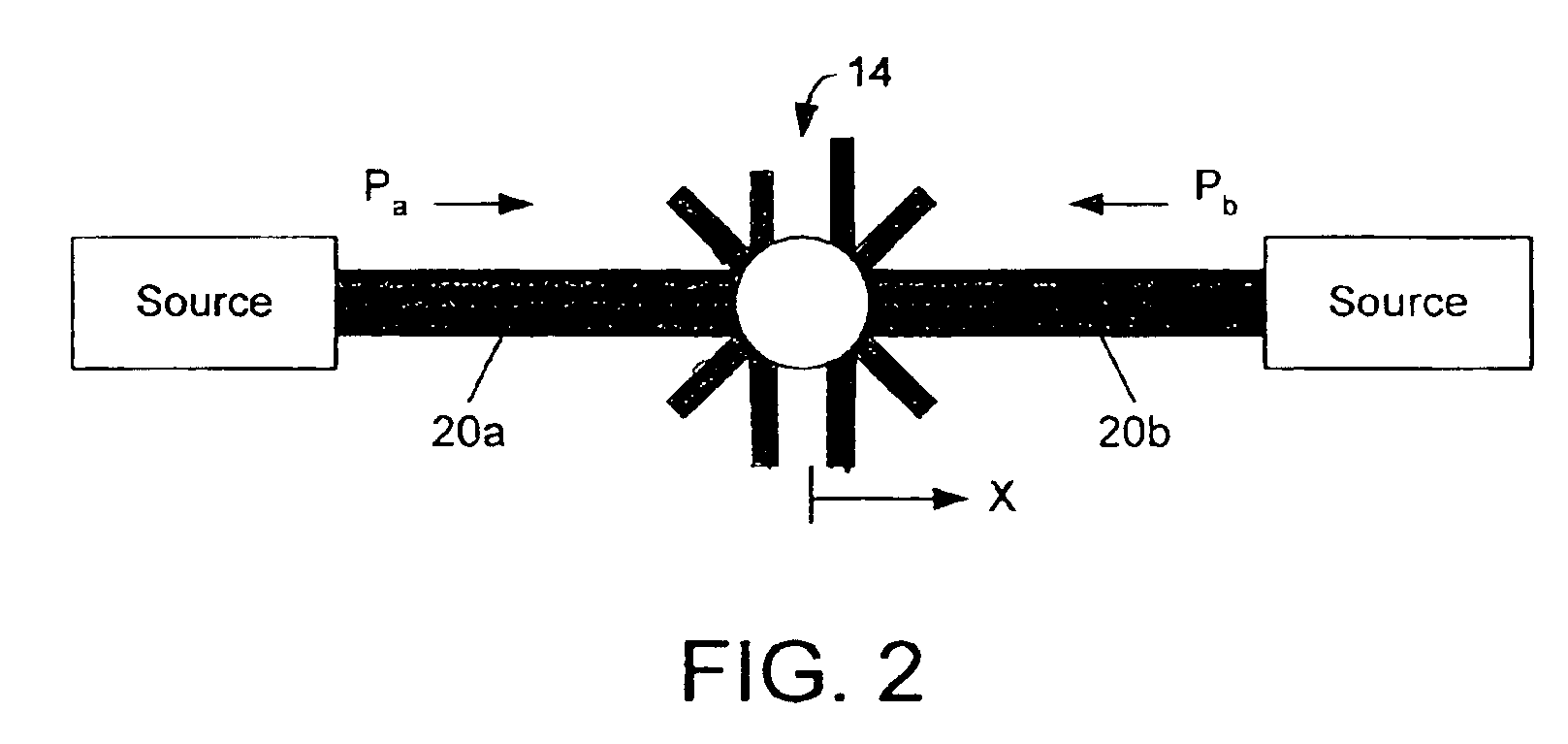
An optical accelerometer for detecting an acceleration of a proof mass includes a source of optical radiation for generating a pair of beams of output radiation. The pair of beams of optical radiation exerts radiation pressure on the proof mass, so as to maintain the proof mass in an equilibrium position along a sensing axis. A position detecting system detects a displacement from the equilibrium position of the proof mass along the sensing axis in response to an inertial force acting on the proof mass. A modulator adjusts the intensity of each one of the pair of beams, so as to restore the proof mass to the equilibrium position along the sensing axis. The difference in the adjusted intensities of each one of the pair of beams is representative of the acceleration, resulting from the inertial force, of the proof mass along the sensing axis.
Owner:CHARLES STARK DRAPER LABORATORY
VPCE (vapor phase catalytic exchange) static performance testing method
The invention discloses a VPCE (vapor phase catalytic exchange) static performance testing method. The method comprises the following steps: S1, putting a catalyst to be detected and analyzed in a reaction tank, and closing; S2, vacuumizing a reaction vessel: washing several times, and then vacuumizing the reaction vessel; S3, separating a mixing tank and the reaction tank to form independent spaces; and introducing pure hydrogen into the mixing tank, and injecting deuterium-containing water into the mixing tank; S4, regulating a temperature control device, so that the temperature of the reaction vessel reaches a preset temperature value; monitoring a pressure sensor and a temperature sensor of the mixing tank and the reaction tank; when the temperature and pressure from the both reach preset requirements, enabling the mixing tank to communicate with the reaction tank; and reacting deuterium-containing vapor and hydrogen gas in the presence of a hydrophilic catalyst; and S5, sampling and detecting: sampling from the reaction tank at set intervals, detecting and analyzing. The VPCE static performance testing method disclosed by the invention can accurately acquire the kinetic curve, equilibrium constant, separation factor and other parameters of hydrogen-water isotope catalytic exchange reaction.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
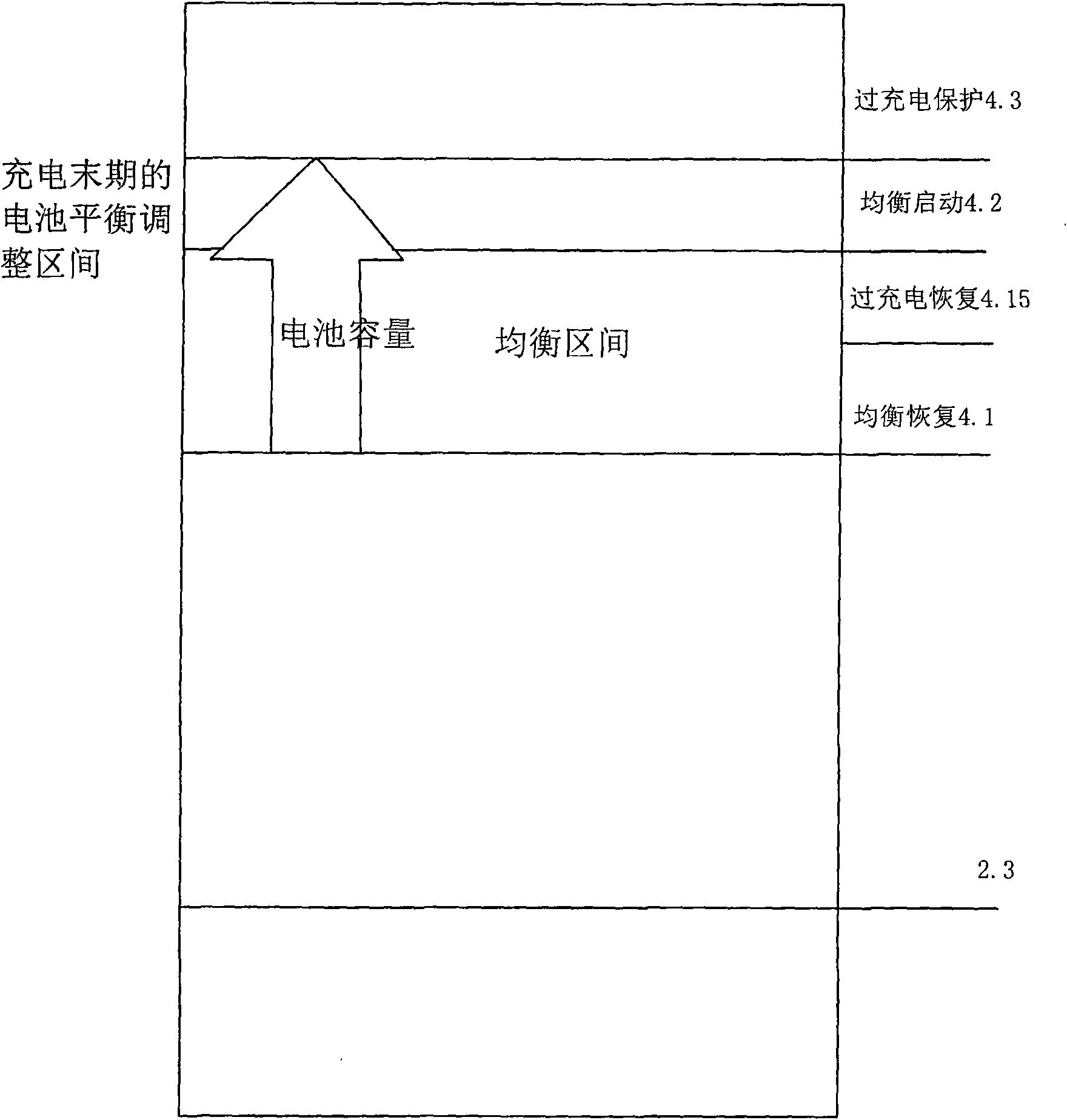
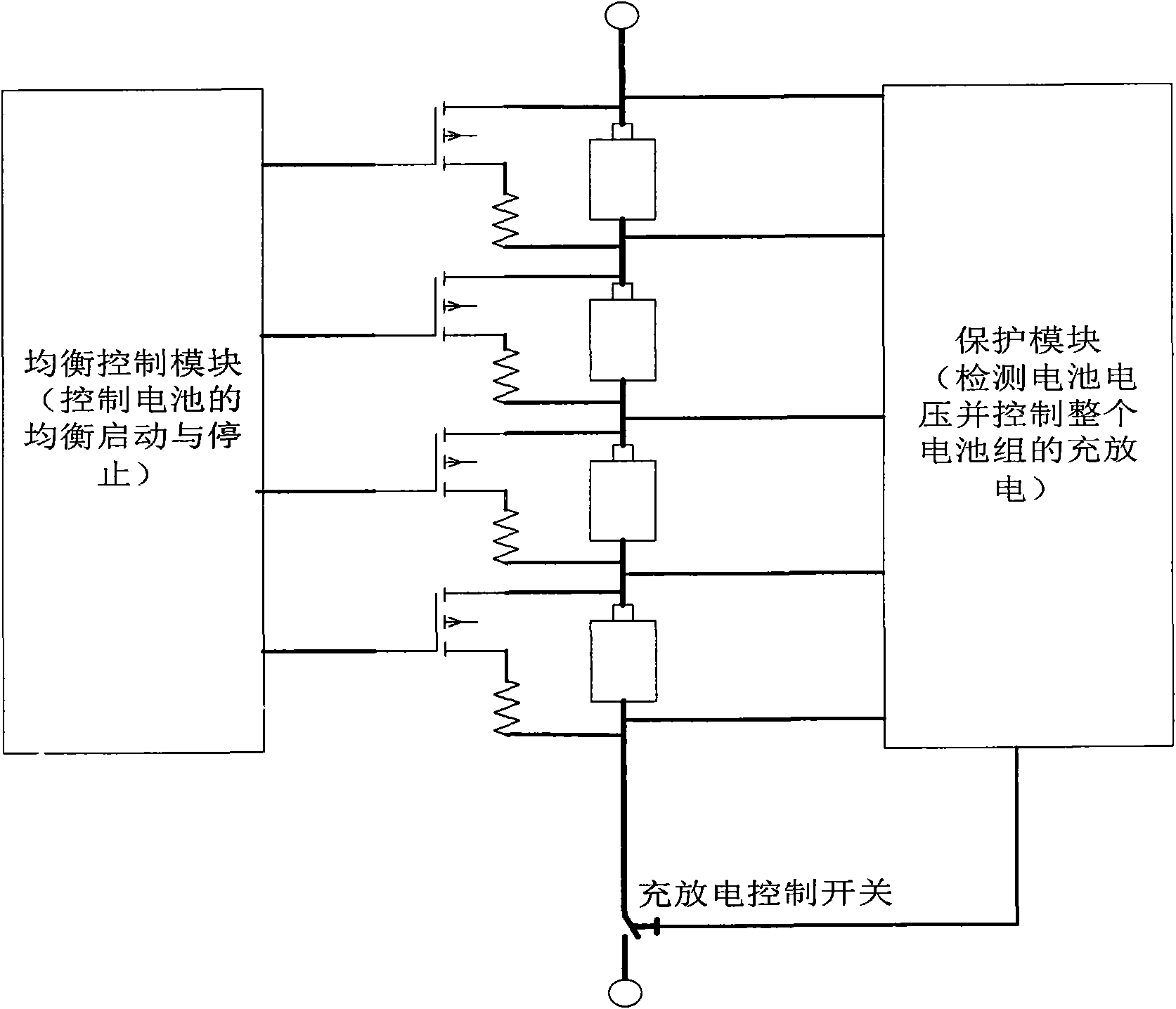
Lithium battery pack charge capacity equilibrium control method and protective device thereof
InactiveCN101599658ATime equalizationShorten charging timeBatteries circuit arrangementsElectric powerEquilibrium constantEquilibrium control
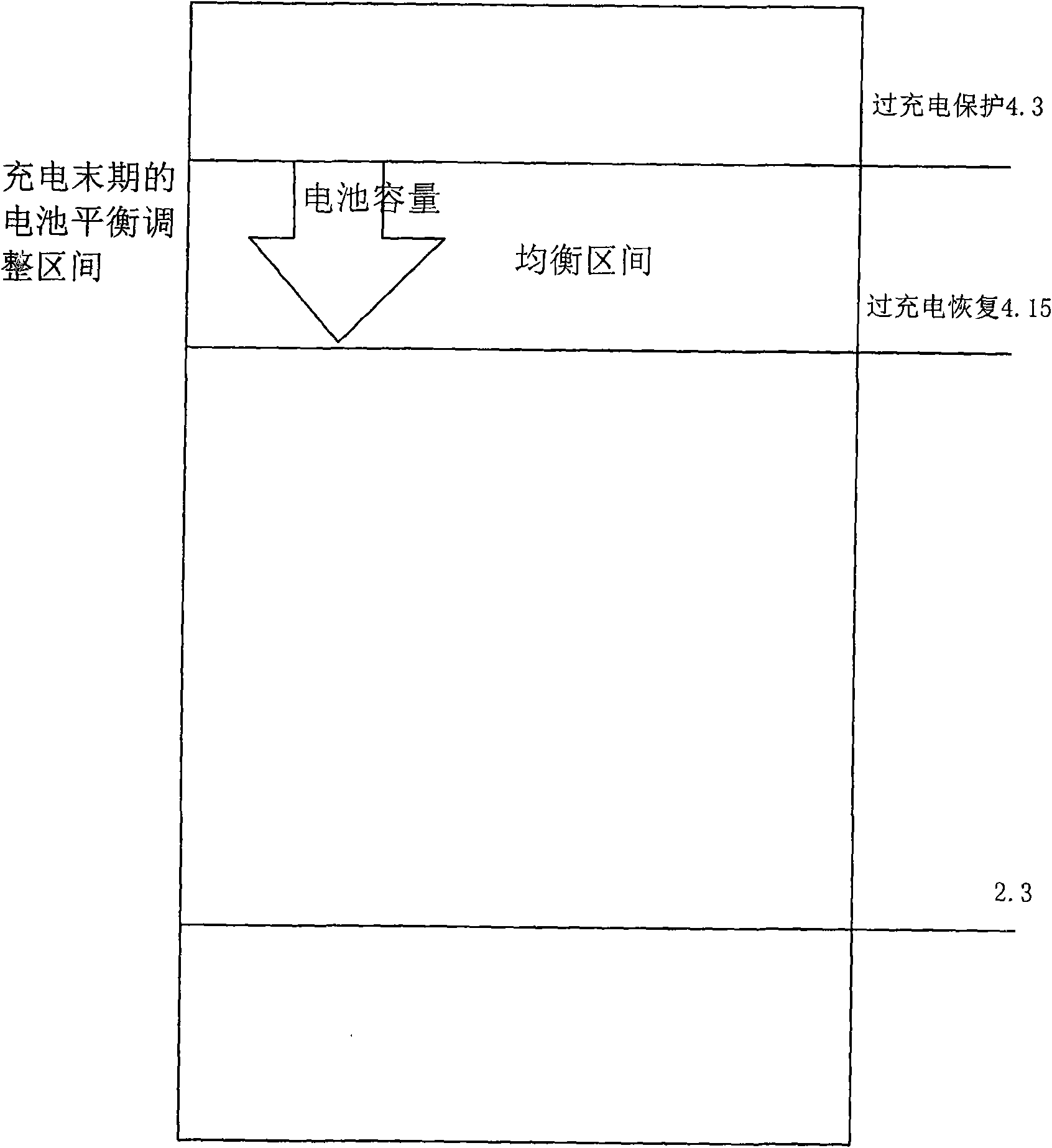
The invention provides a lithium battery pack charge capacity equilibrium control method, which is characterized in that in the charging process, two sampling points are added in the charge equilibrium interval which is between the overcharging protection voltage and equilibrium recovery voltage; the two sampling points are respectively equilibrium starting voltage and overcharging recovery voltage; the equilibrium process acts in both the equilibrium starting and the equilibrium stopping intervals; in the equilibrium stage, a charger charges the battery pack continuously. The method is realized by a charging equilibrium circuit; as the battery protection IC in the invention is adopted, the charging process can equilibrate longer time, thus not only the equilibrium energy is improved for at least two times, but also the charging time is shortened.
Owner:BEIJING HUADA ZHIBAO ELECTRONICS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com