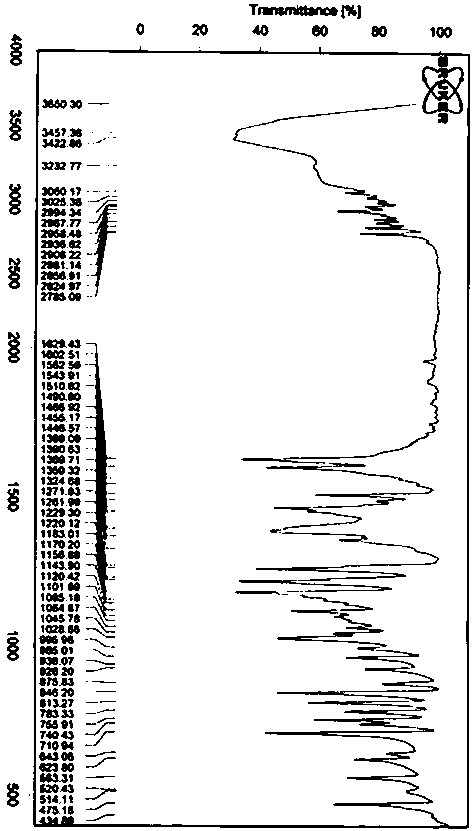
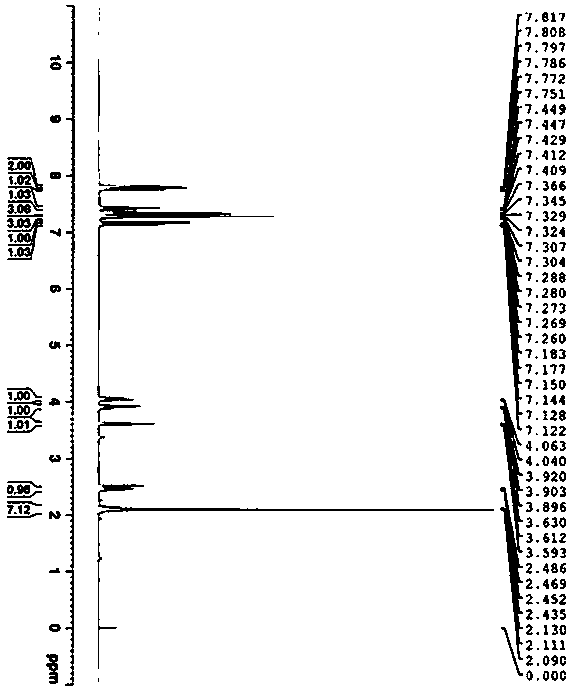
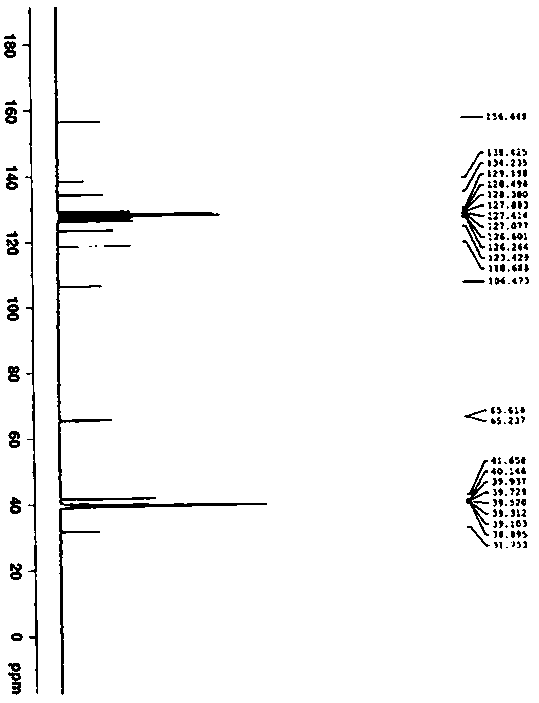
Preparation and detection method of dapoxetine hydrochloride isomer impurities
A technology for dapoxetine hydrochloride and isomers, which is applied in the field of preparation and detection of isomer impurities of dapoxetine hydrochloride, can solve problems such as not being unanimously recognized by people, and achieve mild reaction conditions and stereoselectivity High, simple synthesis process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Step 1) Preparation of Boc-(S)-α-[2-(2-naphthyloxy)ethyl]benzylamine (Ⅵa)
[0043]Add 100mL dimethylformamide to a 500mL three-necked reaction flask, stir, add 20.0g (79mmol) of Boc-(S)-3-amino-3-phenylpropanol to dissolve, and control the internal temperature at 10~30°C under nitrogen protection. Slowly add 4.1g (103mol) of 60% sodium hydride at ℃, and after the degassing becomes gentle, add 11.6g (79mol) of 2-fluoronaphthalene, raise the temperature to 60~70℃ for 5~10 hours, and cool the reaction solution to 20~ 40°C, add 300mL purified water to quench the reaction, extract twice with 200mL ethyl acetate, combine the organic layers, wash twice with 200mL saturated brine, concentrate the organic layer under reduced pressure at 40~50°C, and obtain 26.4g after concentration, namely (Ⅵa ), HPLC purity 91.8%, yield 80.7%.
[0044] Step 2) Preparation of Boc-(R)-α-[2-(1-naphthyloxy)ethyl]benzylamine (Ⅵb)
[0045] Referring to the method of Example 1, 20.0g (79mmol) Boc-(S...
Embodiment 2
[0049] Step 1)~Step 2) Same as above;
[0050] Preparation of (R)-N,N-dimethyl-α-[2-(1-naphthyloxy)ethyl]benzylamine (Ⅶb)
[0051] Step 3) Referring to the method of step 3) in Example 1, feed 25.0g of Boc-(R)-α-[2-(1-naphthyloxy)ethyl]benzylamine to obtain 17.2g of off-white solid powder, Namely (R)-N,N-dimethyl-α-[2-(1-naphthyloxy)ethyl]benzylamine (VIIb), HPLC purity 97.5%, yield 91.2%.
Embodiment 3
[0053] Step 1)~Step 2) Same as above;
[0054] Step 3) Preparation of (R)-N,N-dimethyl-α-[2-(1-naphthyloxy)ethyl]benzylamine (VIIb)
[0055] Add 80mL of isopropanol to a 250mL three-necked reaction flask, stir, and add 15.0g (49mmol) of (S)-N,N-dimethyl-α-[2-(2-naphthyloxy)ethyl]benzylamine , add 6.8g (63mmol) of 34% hydrochloric acid dropwise, heat to dissolve, gradually cool down to -15~-5°C to crystallize for 5 hours, filter, and dry at 60~65°C for 10 hours to obtain 15.6g off-white solid powder, namely ( S)-N,N-Dimethyl-α-[2-(2-naphthyloxy)ethyl]benzylamine hydrochloride (I), HPLC purity 99.8%, yield 92.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com