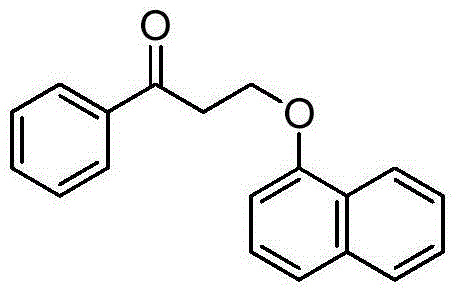
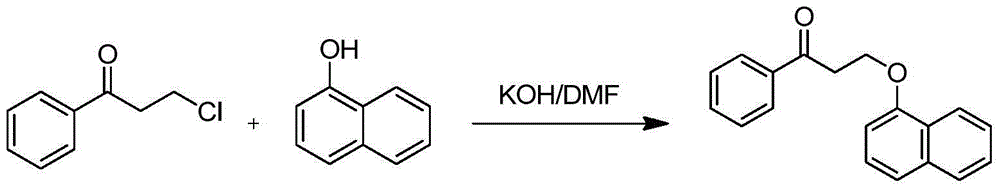
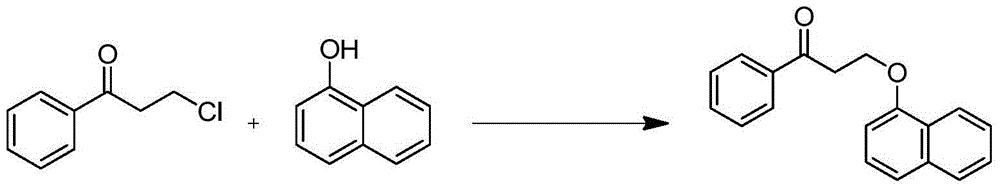
Dapoxetine intermediate and preparation method thereof
A volume and protective gas technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbonyl compounds by hydrolysis, etc., can solve the problems of high price, high production cost, unsuitable for industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Preparation of 2-(2-chloroethyl)-2-phenyl-1,3-dioxolane (compound shown in formula 3)
[0079] Compound 4 (100g, 0.6mol, 1eq) was dissolved in 500mL of dichloromethane (DCM), followed by adding ethylene glycol (133mL, 4eq), trimethyl orthoformate (126.3g about 130mL, 1.19mol, 2eq), mass Concentrated sulfuric acid with a percentage content of 98% (the mass percentage refers to the percentage of the mass of sulfuric acid in the total mass of the concentrated sulfuric acid reagent) (5.8g, 0.1eq). The reaction solution was heated to reflux (45° C.), and after stirring for 6 h, TLC detected that compound 4 disappeared, and the temperature was lowered to cool. Add 20gNaHCO 3 The pH of the solid was adjusted to about 7, and stirred for 10 minutes. After suction filtration, the organic layer was washed with brine (100 mL), dried and concentrated to obtain about 152 g of an oily substance. Add 100mL of isopropanol to the residue, cool overnight to crystallize, and ...
Embodiment 2
[0080] Embodiment 2: Preparation of (3-chloro-1,1-dimethoxypropyl)benzene (compound shown in formula 3)
[0081] Compound 4 (5g, 0.03mol, 1eq) was dissolved in 25mL of dichloromethane (DCM), followed by adding methanol (4.8mL, 4eq), trimethyl orthoformate (6.3g about 6.5mL, 0.06mol, 2eq), mass Concentrated sulfuric acid with a percentage content of 98% (the mass percentage refers to the percentage of the mass of sulfuric acid in the total mass of the concentrated sulfuric acid reagent) (0.29g, 0.1eq). The reaction solution was heated to reflux (42° C.), and after stirring for 6 h, compound 4 disappeared as detected by TLC, and the temperature was lowered to cool. Add 1.5gNaHCO 3 The pH of the solid was adjusted to about 7, and stirred for 10 minutes. After suction filtration, the organic layer was washed with brine (10 mL), dried and concentrated to obtain about 7.2 g of an oily substance. Column chromatography (PE:EA=50:1) of the residue gave about 4.5 g of colorless oil (...
Embodiment 3
[0082] Embodiment 3: Preparation of (3-chloro-1,1-dimethoxypropyl)benzene (compound shown in formula 3)
[0083]Compound 4 (5g, 0.03mol, 1eq) was dissolved in 25mL of dichloromethane (DCM), followed by adding methanol (4.8mL, 4eq), trimethyl orthoformate (6.3g about 6.5mL, 0.06mol, 2eq), mass Concentrated sulfuric acid with a percentage content of 98% (the mass percentage refers to the percentage of the mass of sulfuric acid in the total mass of the concentrated sulfuric acid reagent) (0.29g, 0.1eq). The reaction solution was heated to reflux (about 42° C.), and after stirring for 6 h, compound 4 disappeared as detected by TLC, and the temperature was lowered to cool. Add 1.5gNaHCO 3 The pH of the solid was adjusted to about 7, and stirred for 10 minutes. After suction filtration, the organic layer was washed with brine (10 mL), dried and concentrated to obtain about 7.2 g of an oily substance. Add 30mL of isopropanol to the residue, cool overnight to crystallize, and sucti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com