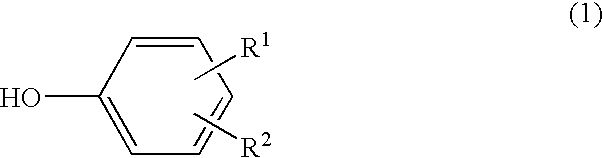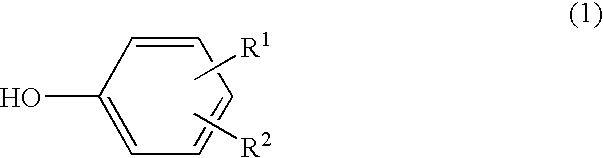Patents
Literature
105 results about "1-Naphthol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C₁₀H₇OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols (both 1 and 2 isomers) are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.
Rhodococcus ruber and application thereof in degradation of phenol pollutants
ActiveCN102604875ABroad-spectrumEfficientBacteriaMicroorganism based processesBenzeneInorganic salts
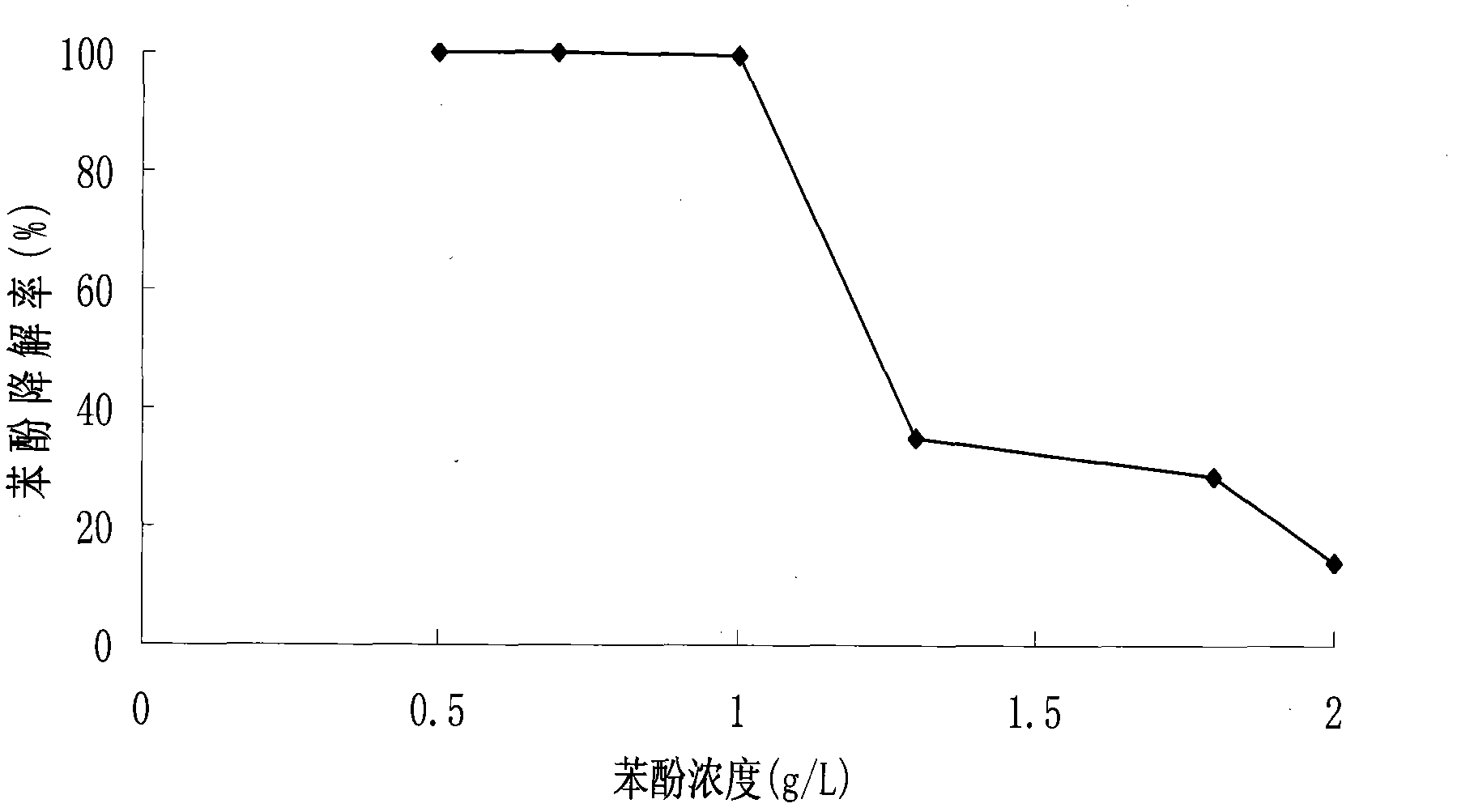
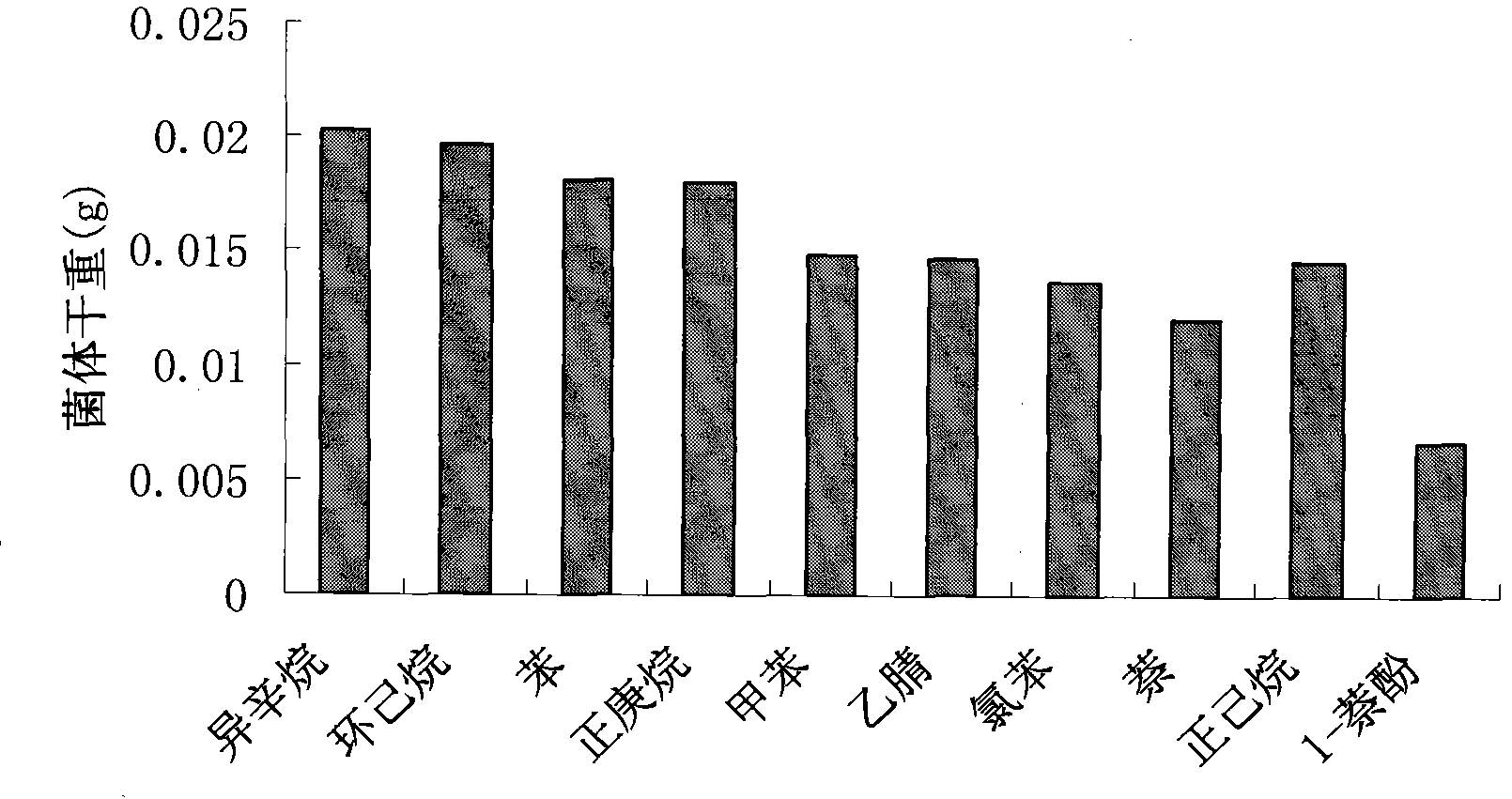
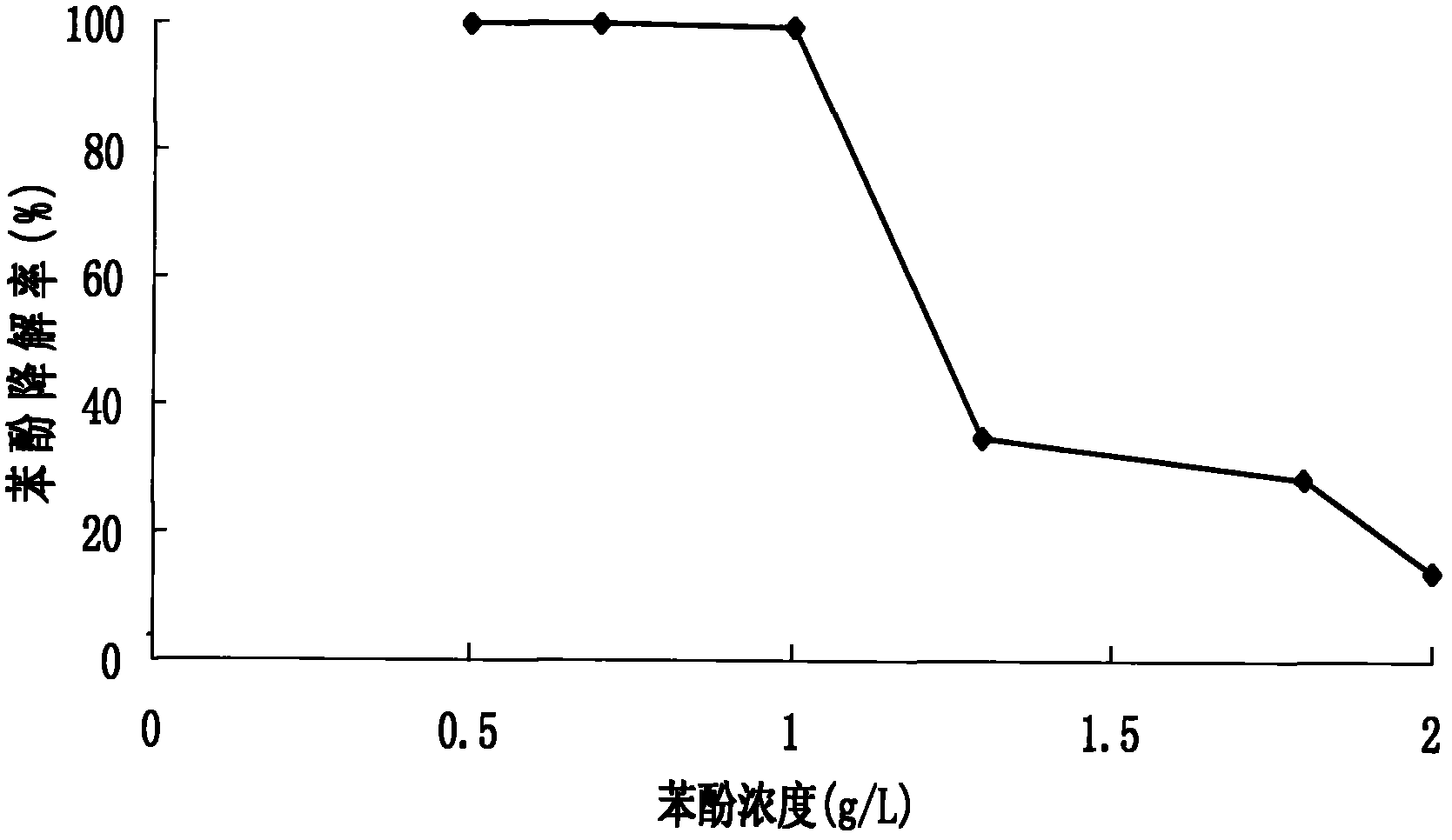
The invention relates to Rhodococcus ruber and application of Rhodococcus ruber in degradation of phenol pollutants. The Rhodococcus ruber is designated as Rhodococcus ruber SD3, which is preserved in a preservation unit appointed by SIPO (State Intellectual Property Office), wherein the preservation unit is a China Center for Type Culture Collection, the preservation date is February 26, 2012, the accession number is CCTCC NO: M 2012035, and the Latin name is Rhodococcus ruber SD3. The Rhodococcus ruber has the following technical effect: after Rhodococcus ruber SD3 in an inorganic salt medium of phenol (phenol concentration is 1.0g / L) is subjected to shaking table vibration culture at 35 DEG C and 200r / min for 72h, the phenol degradation rate is 99.73%. The Rhodococcus ruber SD3 can also degrade substances such as isooctane, cyclohexane, benzene, n-heptane, methylbenzene, acetonitrile, chlorobenzene, naphthalene, n-hexane, and 1-naphthol.
Owner:JIANGXI NORMAL UNIV
Antidepressant oral liquid compositions
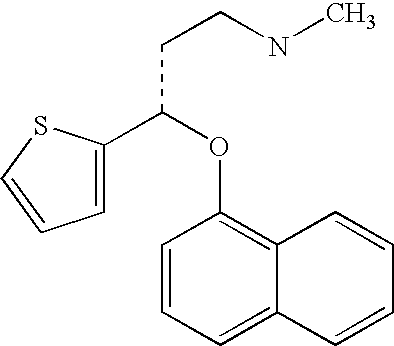
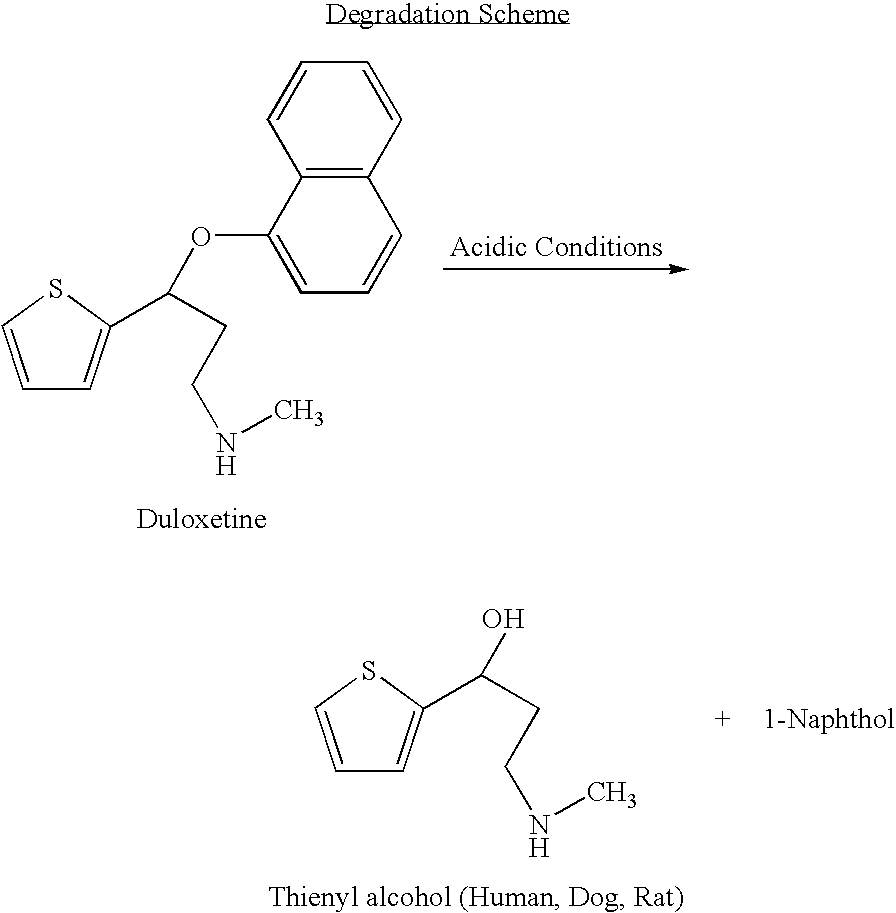
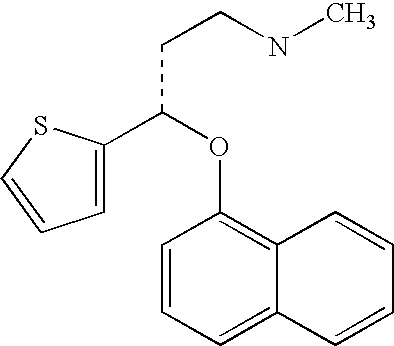
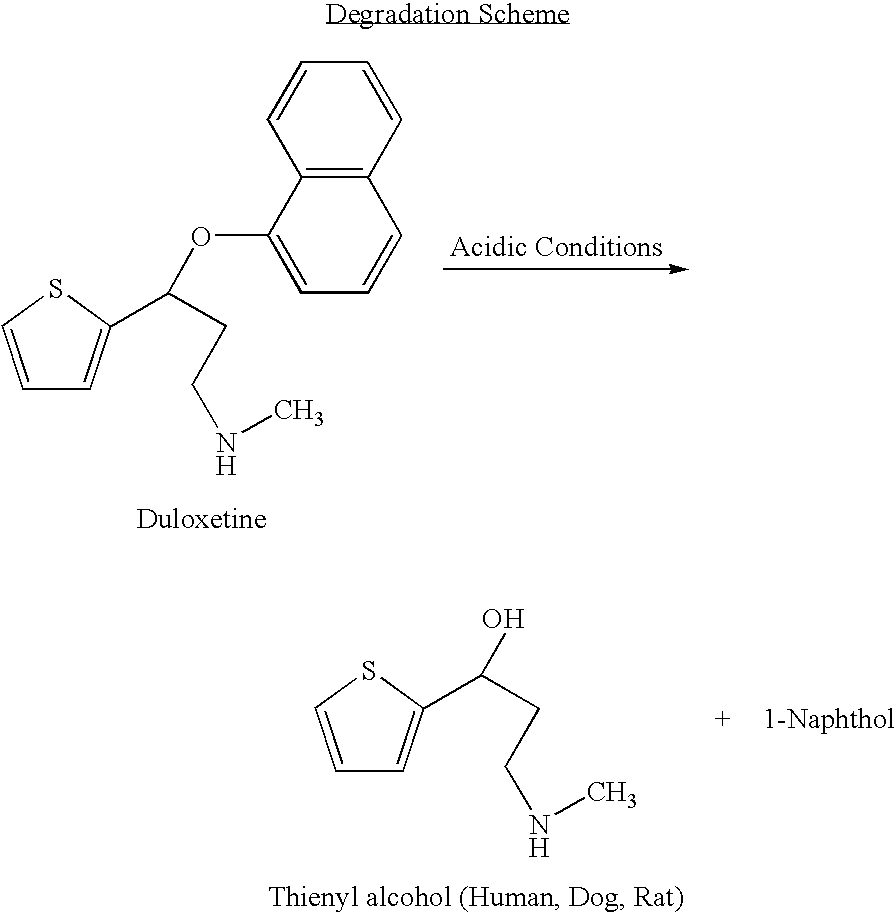
InactiveUS8153824B2Inhibition of reuptakeImprove concentrationBiocideNervous disorderDuloxetinePeripheral neuropathic pain
The invention provides for the first time an oral liquid composition of duloxetine or its pharmaceutically equivalent derivatives like salts, isomers, complexes, polymorphs, hydrates or esters thereof. The duloxetine or its pharmaceutically equivalent derivative is present from about 2 mg to approximately 200 mg; and a buffering agent was used to stabilize the acid sensitive duloxetine. The composition has duloxetine from about 0.1 meq to about 2.5 mEq per mg of duloxetine. The invention further discloses an oral liquid composition of duloxetine or its pharmaceutically equivalent derivative wherein the degradation product 1-Naphthol is less than 0.01%. Also provided is a method for treating of major depressive disorder and or diabetic peripheral neuropathic pain comprising administering to a mammal in need of such treatment a therapeutically effective amount of a composition.
Owner:COMPANY WOCKHARDT THE
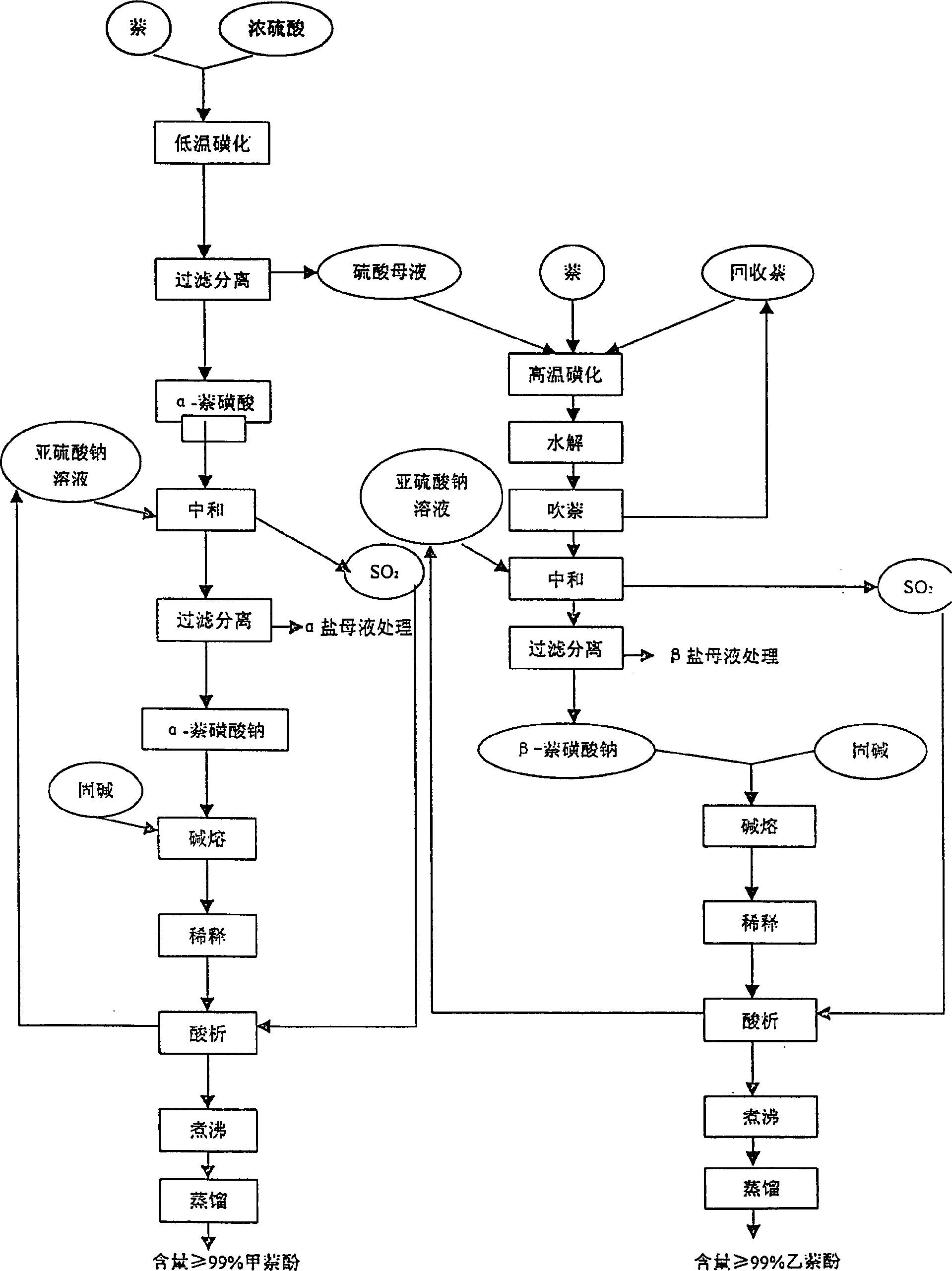
Preparation method of joint production of alpha maphthol and beta naphthol
InactiveCN1513824AAdd stepsLow yieldOrganic chemistryOrganic compound preparation2-Naphthol1-Naphthol
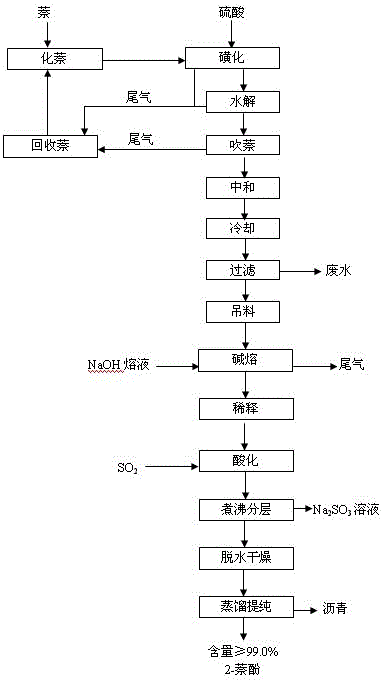
A process for preparing the 1-naphthol and 2-naphthol simultaneously includes sulfonating naphthalene by concentrated sulfuric acid, filtering to obtain alpha-naphthalenesulfonic acid and acidic mother liquid, dripping the mother liquid in fused naphthalene, high-temp sulfonating, hydrolyzing, blowing naphthalene to obtain high-purity beta-naphthalenesulfonic acid solution, respectively neutralizing the alpha-naphthalenesulfonic acid and beta-nephthalenesulfonic acid solution, alkali fusing, acidifying to obtain said two products, and dissolving beta-naphthalenesulfonic acid in sulfuric acid. Its advantages are high output rate and low pollution.
Owner:江苏华达化工集团有限公司
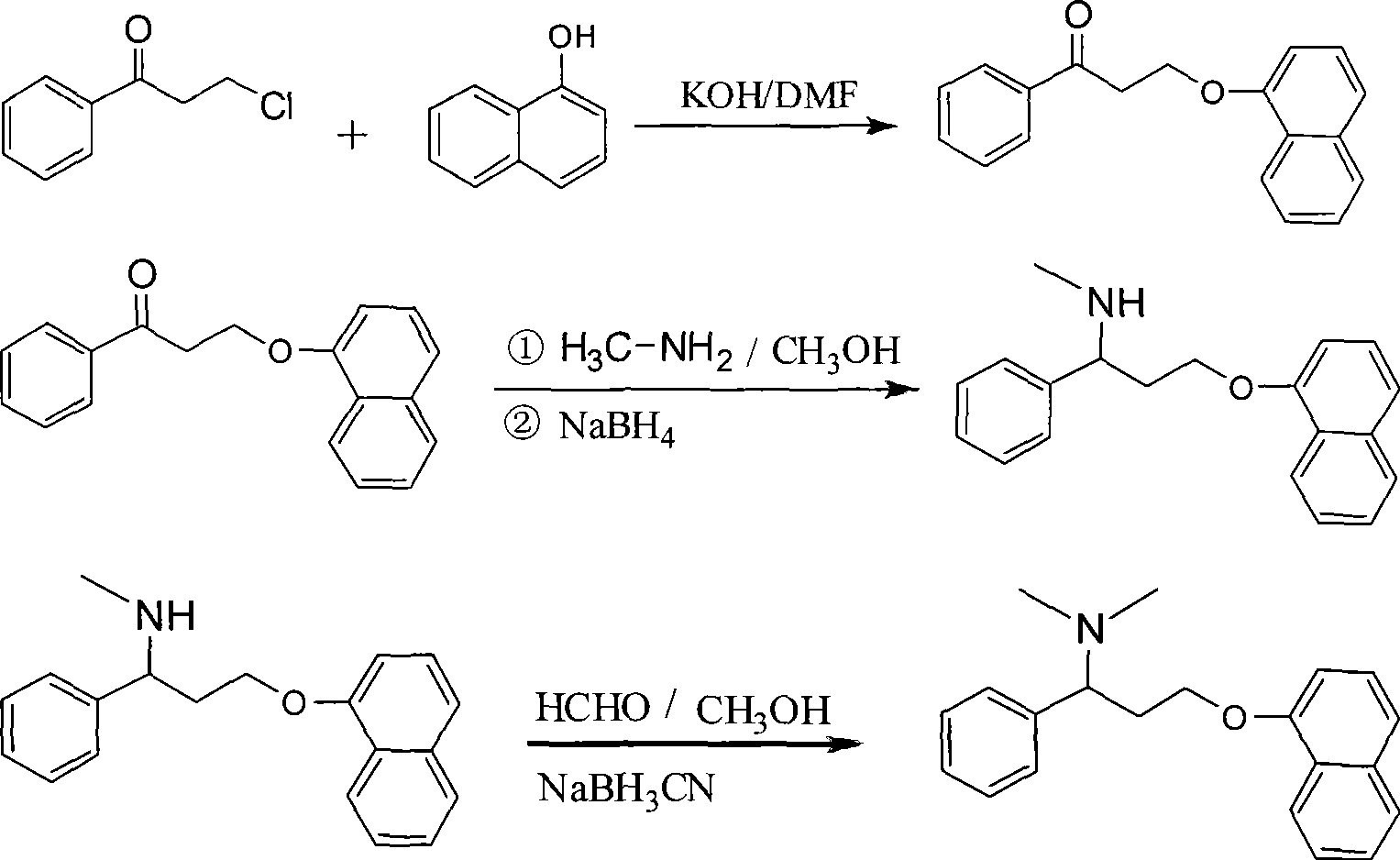
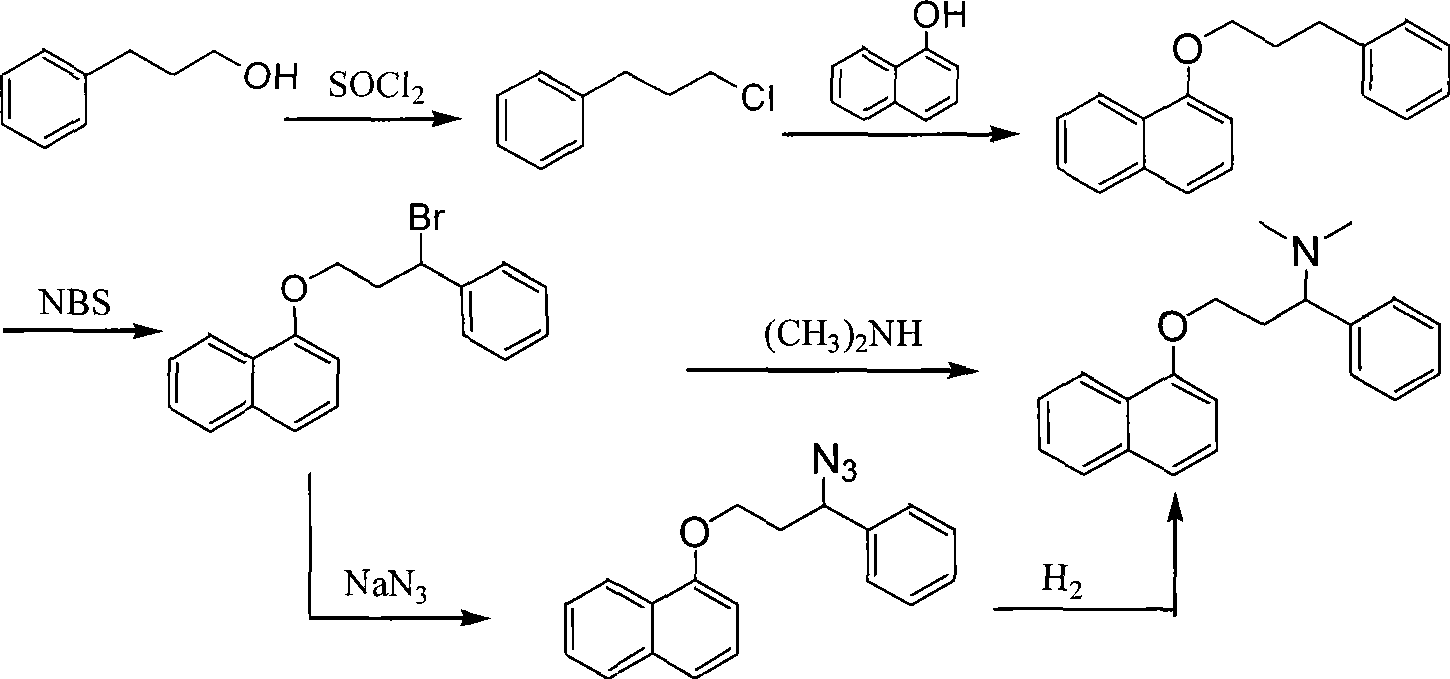
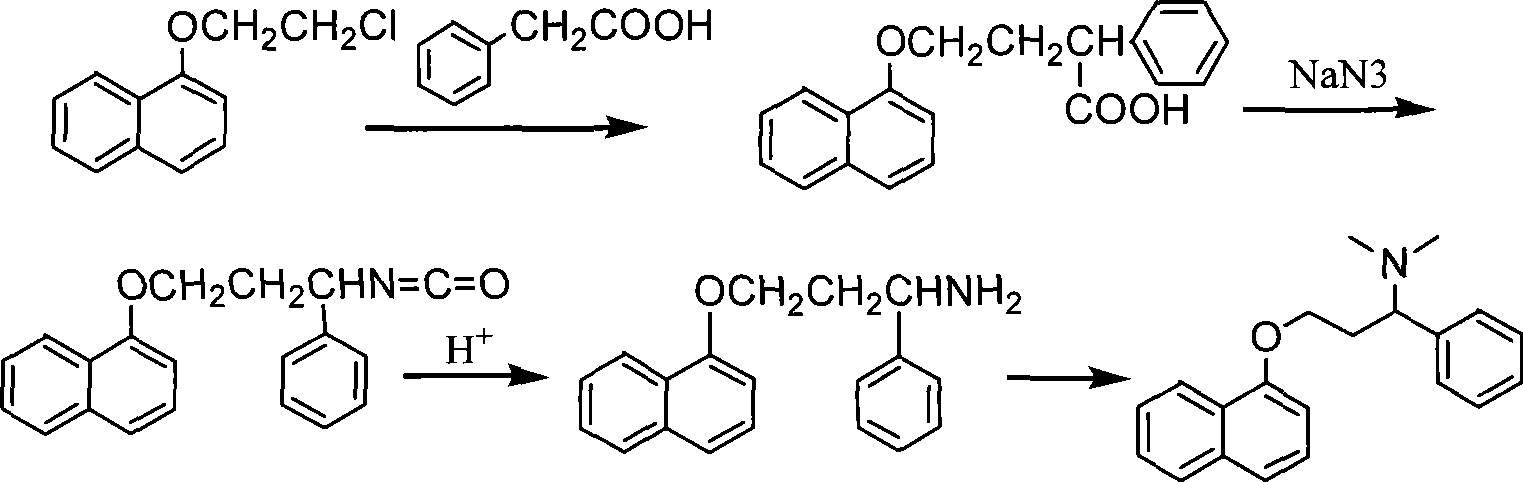
Preparation of N,N-dimethyl-1-phenyl-3-(1-naphthoxy) propanamine
InactiveCN101367739ANon-toxicShort synthetic routeOrganic compound preparationAmino-hyroxy compound preparationChemical industry1-Naphthol
The present invention provides a preparation technology of N, N-dimethyl-1-benzyl-3-(1-naphthyloxies) propylamine, and belongs to the technical field of medical and chemical industry. The present invention solves the problems that the prior synthesis route is long, and the high toxicity, explosiveness, sources or byproducts of raw materials are hard to control. In the preparation technology of the N, N-dimethyl-1-benzyl-3-(1-naphthyloxies) propylamine, 3-chlorine-1-phenyl propanone is used as a starting raw material and is condensed with 1-naphthol, and the N, N-dimethyl-1-benzyl-3-(1-naphthyloxies) propylamine can be prepared through amination, reduction and methylation. The present invention has the advantages that the synthesis route is shortened, and the cost is reduced.
Owner:TAIZHOU VOCATIONAL & TECHN COLLEGE
Cyanide-free silver plating solution additive
The invention relates to a cyanide-free silver plating solution additive which comprises the following components by ratio: 0.1-10g / l of brightener, 5-10g / l of leveling agent, 100-600g / l of complexing agent and the balance of plasma water, wherein the brightener is one or mixture of more in nitrogen-containing compound, triazole, benzotriazole, 2-hydroxypyridine, pyridine, 22 dipyridyl, 1, 10-phenanthroline, triethylene tetramine and diethylene triamine according to any ratio; the leveling agent is one or mixture of more in aromatic hydrocarbon compounds, naphthalene, 1-methylnaphthalene, 1, 4-naphthoquinone and 1-naphthol according to any ratio; the complexing agent is one or mixture of more in disodium ethylenediamine tetraacetate, niacin, aminosulfonic acid and potassium pyrophosphate according to any ratio. The cyanide-free silver plating solution additive has the beneficial effects that the plating solution is stable, low in toxicity and good in dispersing ability; the obtained plating layer is bright and fine as well as good in binding force; the technology adopts the environment-friendly organic additive which does not contain heavy metal and sulfide; the plating layer is good in corrosion resistance. Furthermore, the cyanide-free silver plating solution additive can be directly used for parts such as brass, copper, chemical nickel and the like, preplating is not needed, and the binding force is also guaranteed.
Owner:HANGZHOU WIN WIN TECH CO LTD
Nitrite quick detecting reagent box and application thereof
InactiveCN1936548AChange detectabilityTime consuming to changeMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsSulfanilic acidNitrite
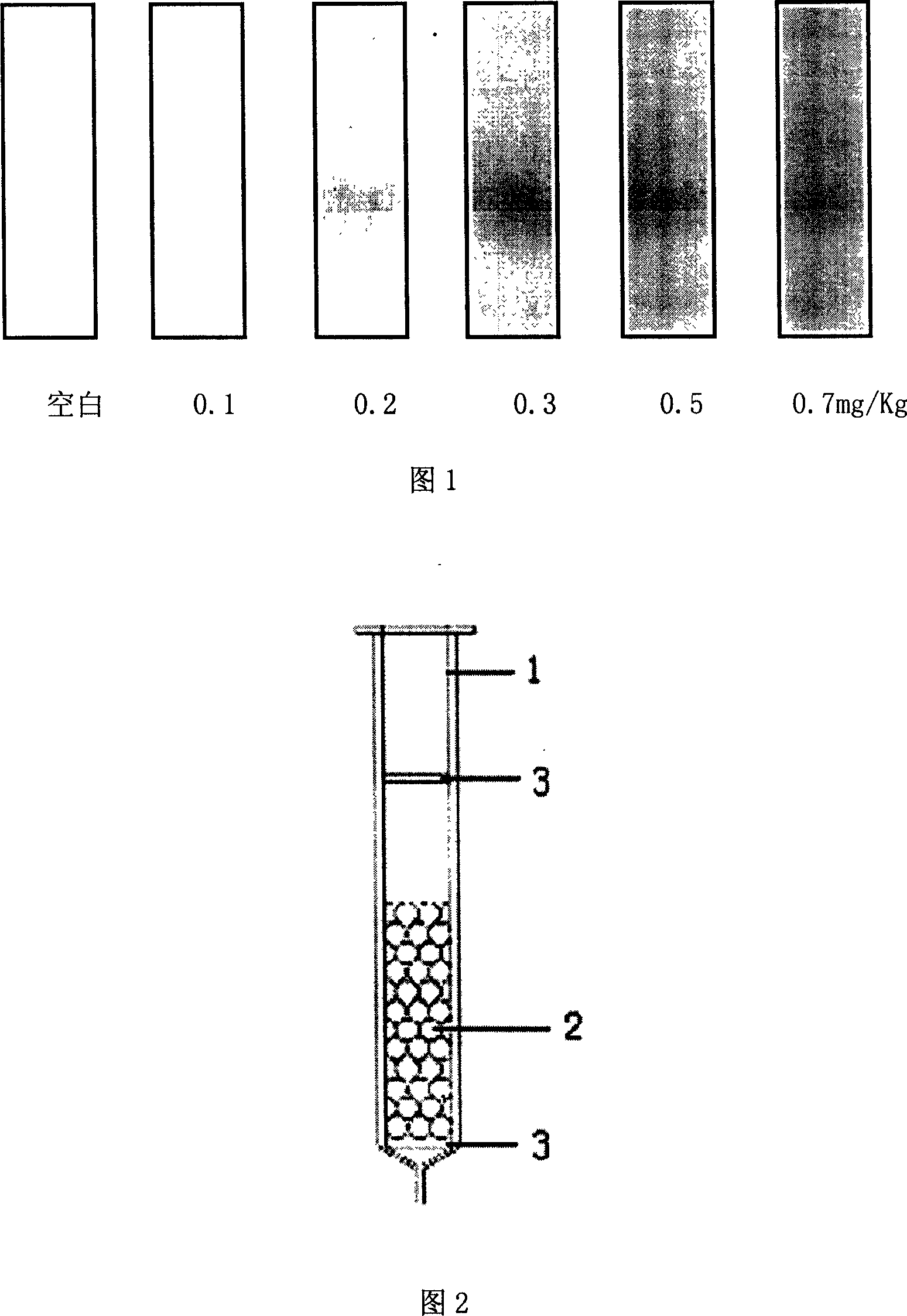
This invention relates to a quick test reagent box and its application of nitrite composed of a reaction post, colorimetric cylinder, 1-naphthol, sulfanilic acid hydrochloric acid solution, potassium ferrocyanide, zine acetate, borax saturated solution and a comparison card. The test method includes: preparing a sample process fluid to be processed in chromogenic reaction to compare with the comparison card and determine the content of nitrite in the sample to judge if it has exceeded the target by transformation, which can semiquantitatively test nitrite in foodstuff and the lowest detection limit is 0.1mg / kg.
Owner:TIANJIN UNIV OF SCI & TECH
Highly sensitive pH test paper and preparation method thereof
ActiveCN102519959AUniform and relatively firm adsorptionStrong adsorptionMaterial analysis by observing effect on chemical indicatorBromothymol bluePhenolphthalein
The invention relates to a piece of highly sensitive pH test paper and a preparation method thereof, wherein a developing strip is in the form of a piece of pH body paper, which is dipped in a reagent solution and then dried by hot air at constant temperature; the reagent solution contains an indicator; the formula of the indicator contains bromothymol blue, thymol blue, metanil yellow, m-methyl red, bromocresol green, nitrazine yellow, cresol red, phenolphthalein, para-nitrophenylazosalicylate sodium, titan yellow, fluorescent pigment orange red, 5% 4-nitrobenzenediazo-1-naphthol-3.6-sodium disulfonate solution and 30% dissolved ethanol aqueous solution; and the formula of the indicator further contains an aqueous colour fixing agent, a cotton wooden fibre mildew preventive, a special paper mildew preventive and a buffer neutralizer formed by modulating sodium hydroxide and phosphoric acid. The preparation method comprises the following steps of: adjusting the reagent solution till a pH value is up to 6-7.5, uniformly wetting the pH body paper, and drying through hot air at constant temperature. According to the invention, the reagent solution can be adjusted, so that the pH value is up to 6-7.5; steady and precise high sensitivity of the pH1-14 test paper is effectively increased; and the test paper is convenient and simple to use.
Owner:上海馨晟试化工科技有限公司
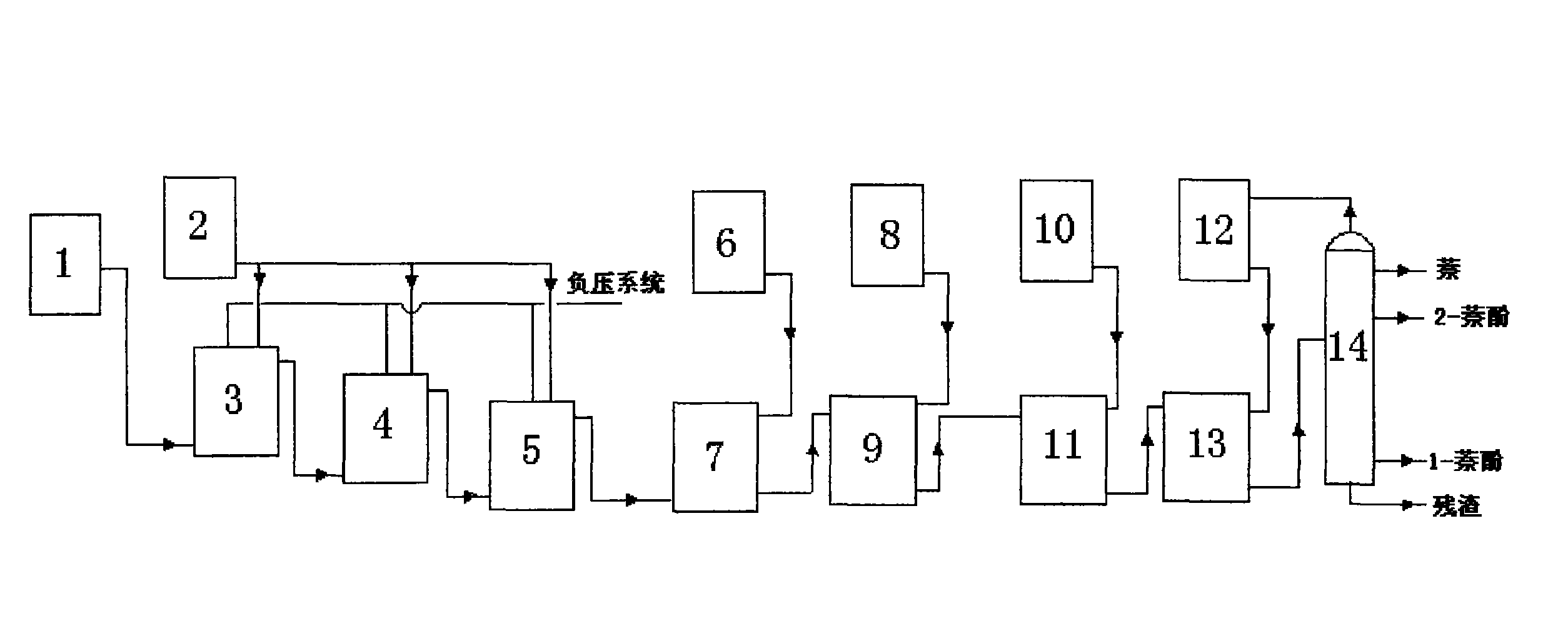
Method for cooperatively producing 1-naphthol and 2-naphthol from naphthalene sulfonation product by virtue of direct alkali fusion
ActiveCN104693009AAlkali fusion reaction goes wellReduce contentOrganic chemistryOrganic compound preparationMolten state2-Naphthol
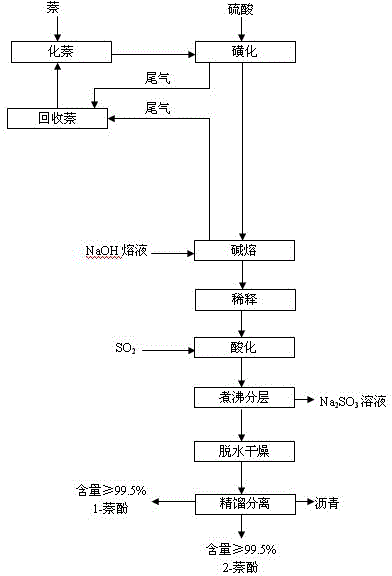
The invention discloses a method for cooperatively producing 1-naphthol and 2-naphthol from a naphthalene sulfonation product by virtue of direct alkali fusion. The method comprises the following steps: performing multiple sulfonation reactions on naphthalene and concentrated sulfuric acid, directly performing an alkali fusion reaction on the obtained liquid sulfonation product and sodium hydroxide in a molten state, and then diluting, acidifying, boiling for layering, dehydrating and drying, rectifying and separating, thereby obtaining high-purity 1-naphthol and 2-naphthol simultaneously. The steps of hydrolyzing, naphthalene blowing, neutralization, cooling and crystallization and filtration of sulfonation products, and feeding for performing alkali fusion on a water-containing filter cake at a normal temperature in the existing process are avoided; no filtration wastewater difficult to treat is not generated anymore, the energy source and the resources are saved, the yield is increased and the operating environment is improved; as a result, the problems of three-waste treatment during naphthol production are thoroughly solved from the production process.
Owner:肖刚学
Process for preparing still release agent
The present invention relates to the preparation of chemical product, and especially the preparation process of chemical assistant for polyvinyl chloride resin production. The still release agent is prepared with thiourea dioxide as reducing decolorant and through the condensation of 1-naphthol and formaldehyde under alkali environment. The preparation process is simple and low in cost, and the prepared still release agent has high still releasing capacity and no coloring.
Owner:JIANGSU JIAHUA ADVANCED MATERIALS TECH
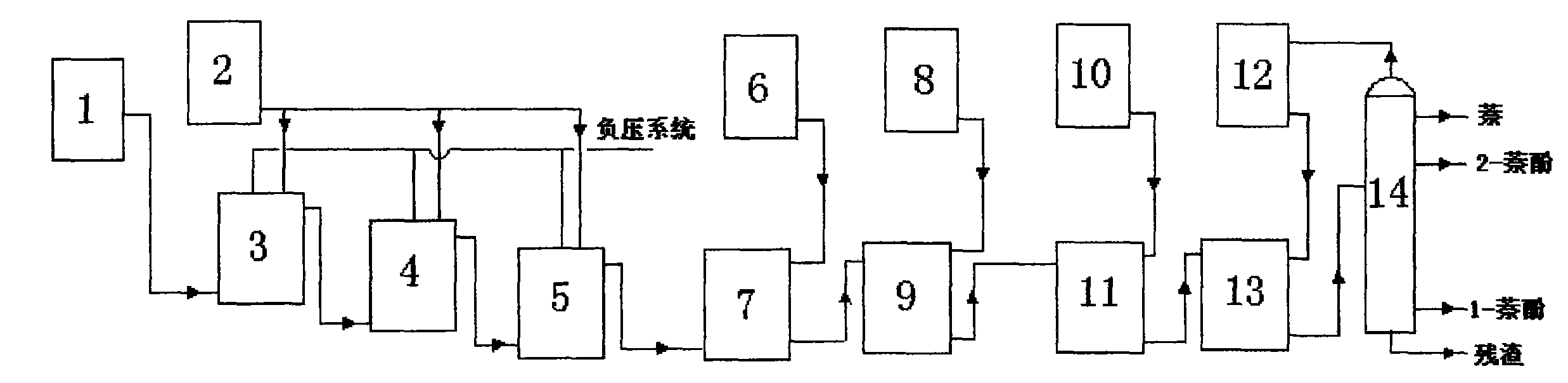
Novel process for efficiently and continuously synthesizing 2-naphthol
ActiveCN101781172AHigh synthesis efficiencyMany steps in the separation processOrganic chemistryOrganic compound preparationMelting tank2-Naphthol
The invention discloses a novel process for efficiently and continuously synthesizing 2-naphthol, which comprises the following steps of: (A) putting refined naphthol from a naphthol-melting tank (1) into a prime kettle of a sulfonation reaction kettle, respectively putting sulphuric acid into various levels of the sulfonation reaction kettle from a sulphuric acid-weighting tank (2) for a sulfonation reaction for 2-6h and reducing the pressure of the sulfonation reaction kettle so as to remove water generated by the reaction; (B) guiding a sulfonated product in the step (A) into a neutralizing tank (7), directly neutralizing with diluted alkali, keeping impurities in the sulfonation reaction, primarily evaporating to dewater and directly guiding into an alkali melting pot (9) for an alkali-melting reaction; (C) acidifying an alkali-melting reaction product in an acidifying tank (11) and extracting 2-naphthol and 1-naphthol from the alkali-melting reaction product in an extracting tower (13) with an extracting agent; and (D) rectifying an organic phase after extracting the naphthol in a rectification tower to recycle the extracting agent and rectifying and separating the 2-naphthol, the 1-naphthol and naphthol without participating the reaction. The invention adopts continuous reaction process design, has simple and convenient process, stable performance and high yield, overcomes the detects of intermittent operation, low synthesis effect, multiple steps for separating 2-naphthol from other impurities, low separation efficiency and much three-waste generation in a sulfonation alkali-melting 2-naphthol synthesis process of the prior art, realizes continuous process, greatly reduces three-waste emission, lessens wastewater by 20 percent than that of the traditional process, greatly improves the 2-naphthol synthesis efficiency and obtains the 1-naphthol as a byproduct.
Owner:QUJING ZHONGYI FINE CHEMICAL INDUSTRY CO LTD
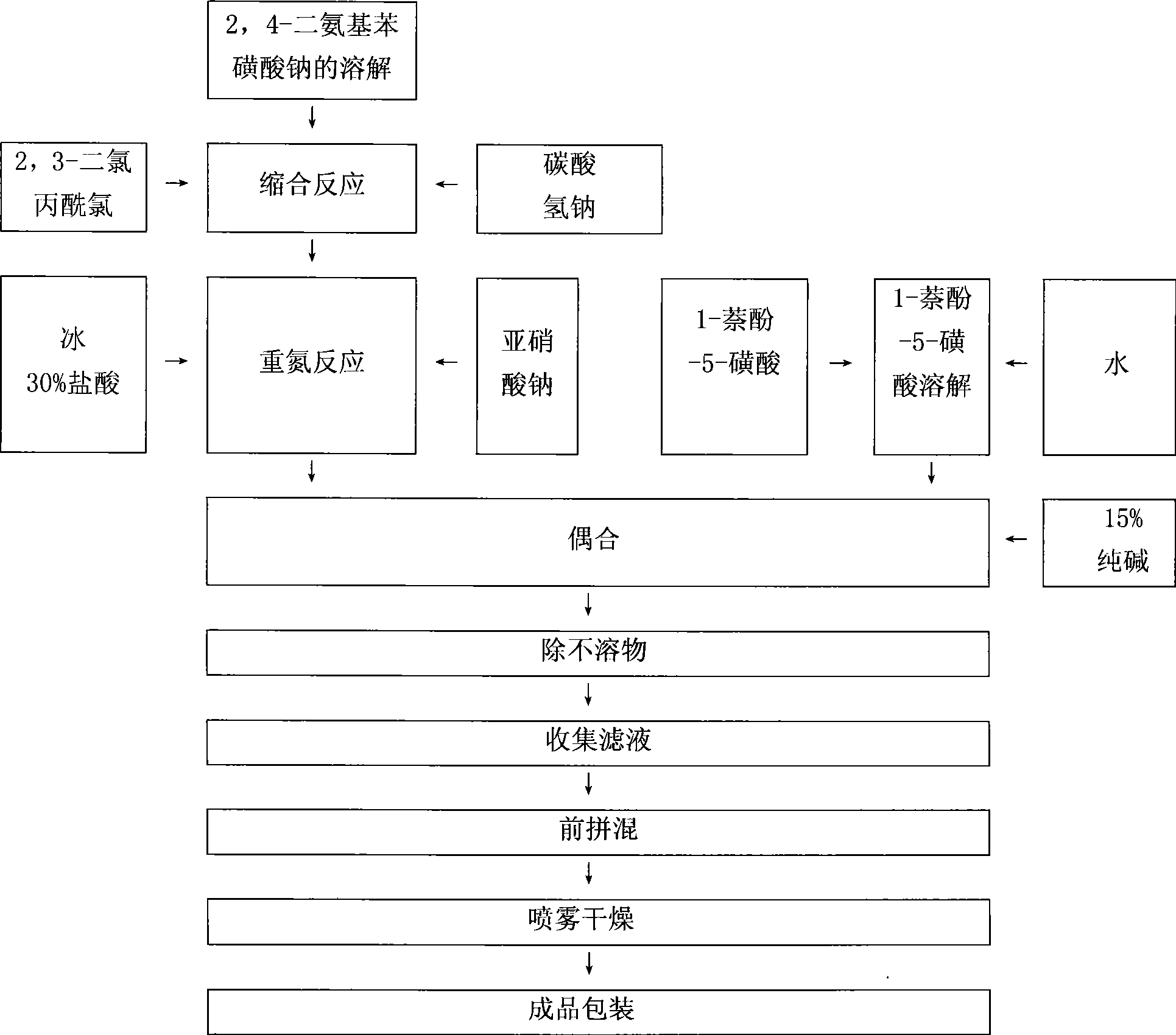
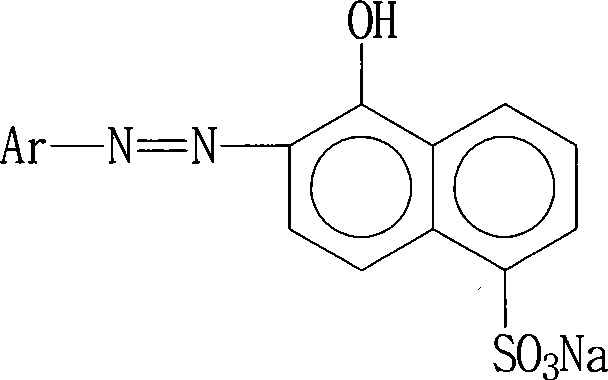
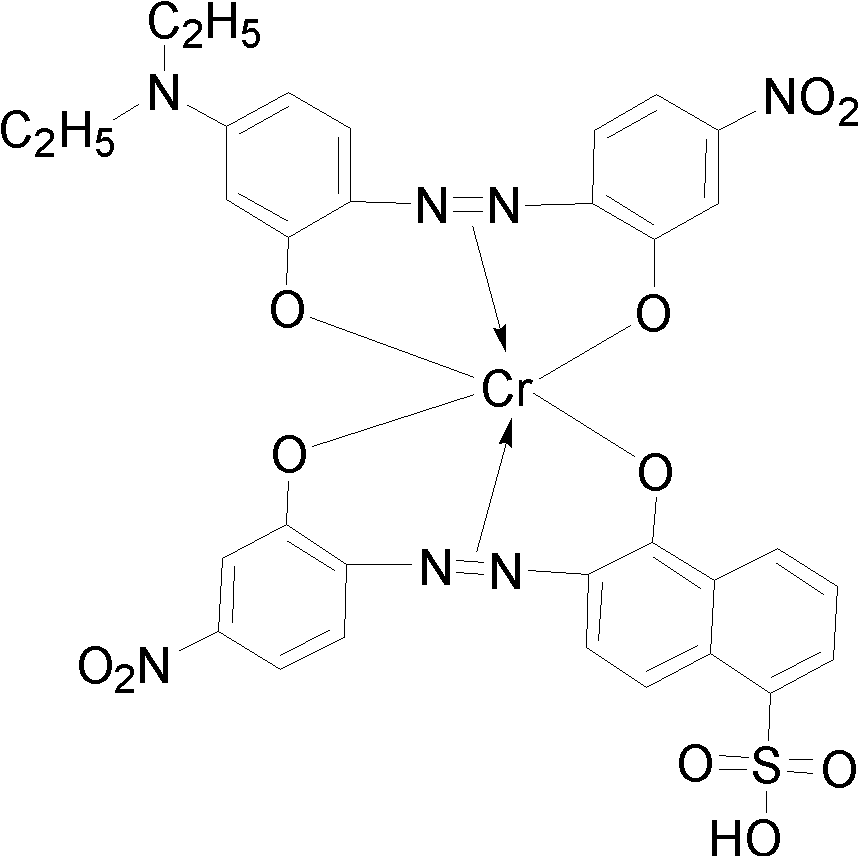
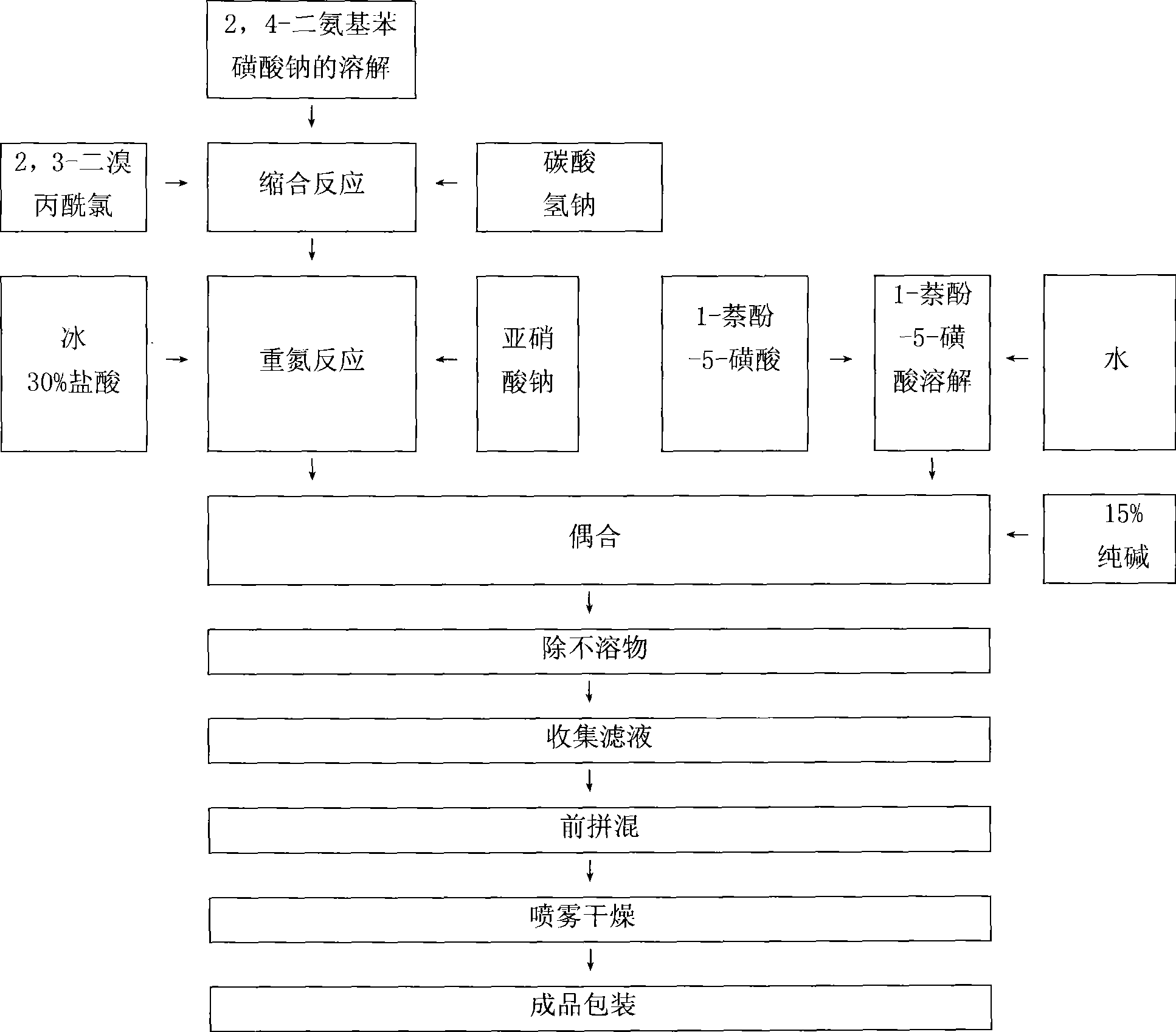
Red reactive dye for fur and preparation thereof
InactiveCN101481523AImprove responseImprove solubilityReactive dyesDyeing processSolubilitySulfonate
The invention discloses a red reactive dye used for fur and a preparation method thereof. The red reactive dye used for fur takes 2, 4-diaminobenzene sodium sulfonate, 1-naphthol-5-sulfoacid, 2, 3-dichloro propionyl chloride as main raw materials. The red reactive dye of the invention is prepared by condensation, diazotization, coincidence, chromatic light adjustment, intensity adjustment, drying and packaging. The red reactive dye used for fur in the invention has relatively high responsiveness, good solubility and bright-colored and beautiful chromatic light; in addition, the light fastness property is relatively good, the exhaustion rate and the color fixing rate are very high. The pre-blending technology and the virgin stock spraying technology are adopted after film processing. Therefore, waste water and waste residue are not generated, greatly contributing to the environmental protection.
Owner:TIANJIN DEK CHEM
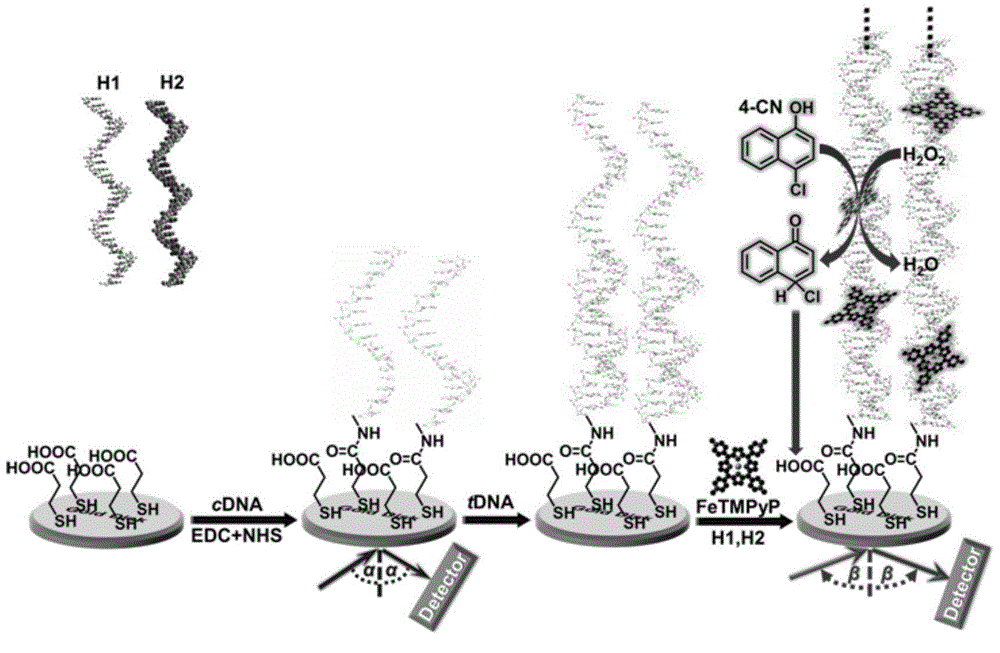
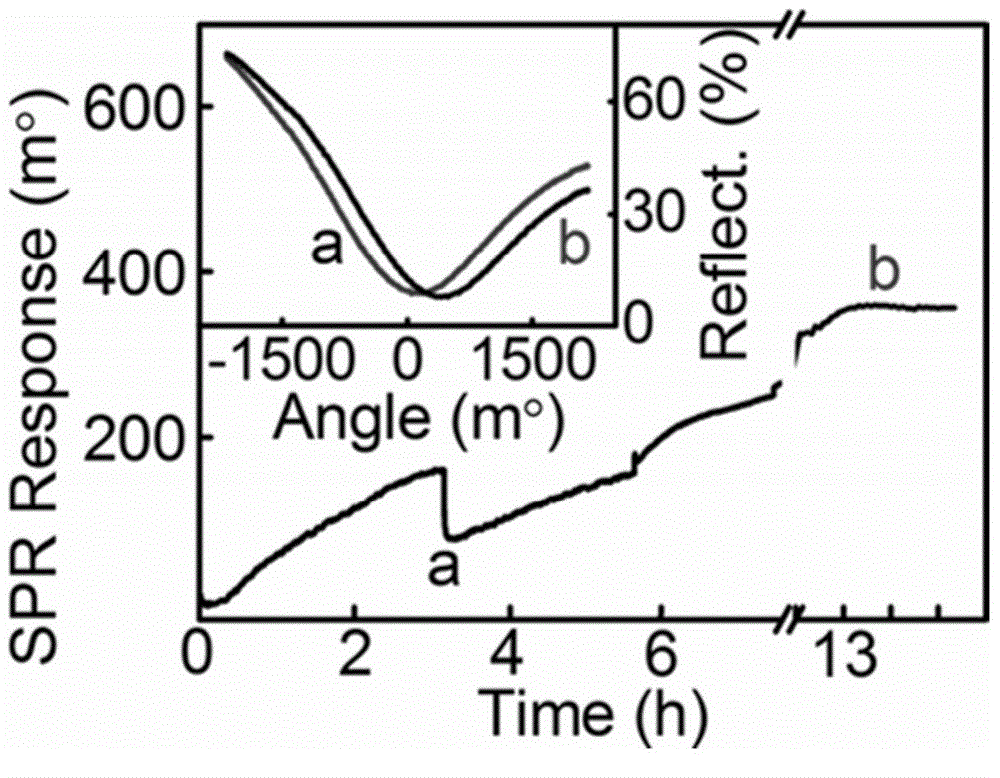
Nucleic acid detection method based on surface plasmon resonance technology
InactiveCN104962633ASensitive detection responseStable in natureMicrobiological testing/measurementEmbedded technologyNucleic acid detection
The invention discloses a nucleic acid detection method based on surface plasmon resonance technology, which belongs to the field of nucleic acid analysis. The nucleic acid detection method is combined by hybrid chain polymerization and a porphyrin groove embedded technology, so as to prepare a porphyrin-dsDNA long-chain polymerization nanoscale efficient biomimetic catalyst, and redox deposition reaction of 4-chlorine-1-naphthol and hydrogen peroxide is catalyzed by utilizing the catalyst, so as to obtain a significant SPR enhanced signal. The nucleic acid detection method has the advantages of high sensitivity, low price, simplicity in preparation, good stability and repeatability and the like when being used for detecting nucleic acid.
Owner:NANJING UNIV OF SCI & TECH
Antidepressant oral liquid compositions
InactiveUS20060079569A1Easy to prepareInhibition of reuptakeBiocideNervous disorderDuloxetinePeripheral neuropathic pain
The invention provides for the first time an oral liquid composition of duloxetine or its pharmaceutically equivalent derivatives like salts, isomers, complexes, polymorphs, hydrates or esters thereof. The duloxetine or its pharmaceutically equivalent derivative is present from about 2 mg to approximately 200 mg; and a buffering agent was used to stabilize the acid sensitive duloxetine. The composition has duloxetine from about 0.1 meq to about 2.5 mEq per mg of duloxetine. The invention further discloses an oral liquid composition of duloxetine or its pharmaceutically equivalent derivative wherein the degradation product 1-Naphthol is less than 0.01%. Also provided is a method for treating of major depressive disorder and or diabetic peripheral neuropathic pain comprising administering to a mammal in need of such treatment a therapeutically effective amount of a composition.
Owner:COMPANY WOCKHARDT THE
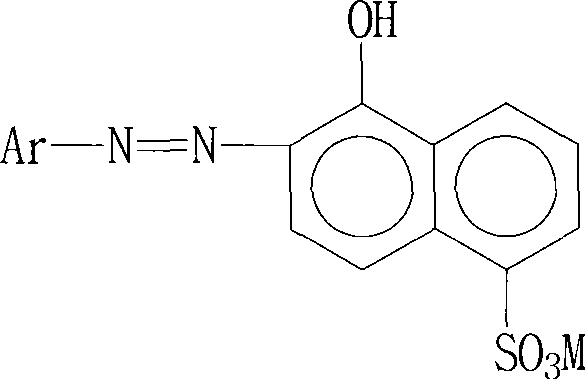
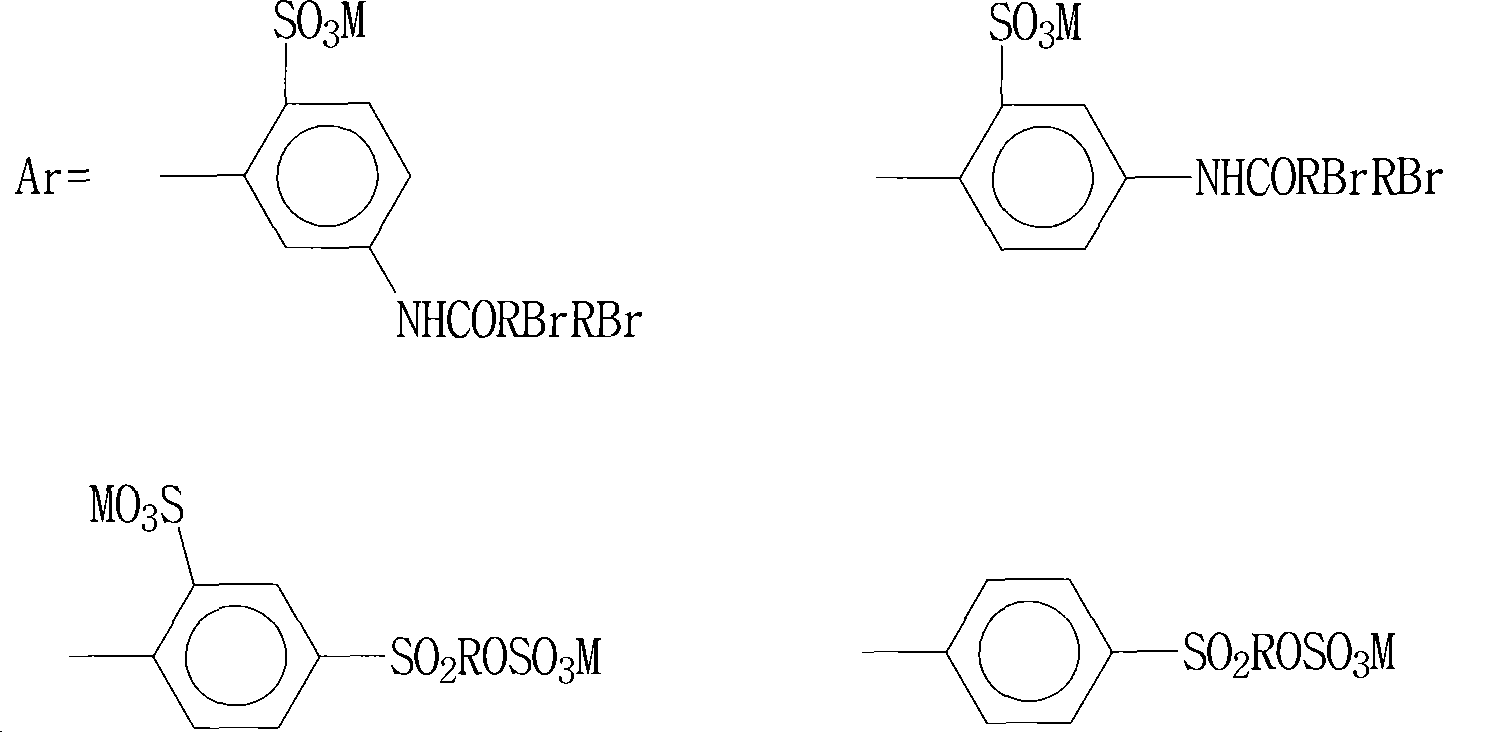
Preparation method of blue dye
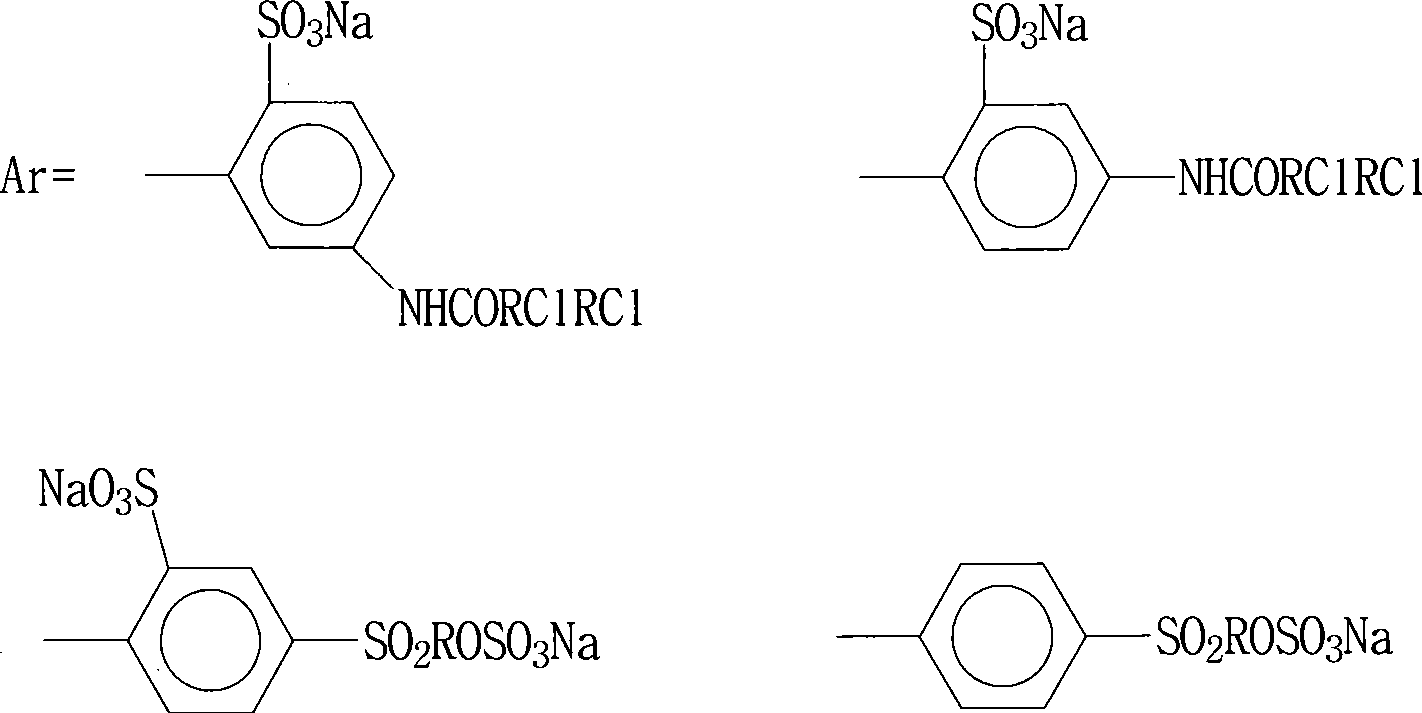
The invention relates to a preparation method of a blue dye. The preparation method provided by the invention comprises two times of diazotization reactions; a first diazotization product is mixed with a 1-naphthol-5-sulfonic acid solution and butanone, such that first coupling is carried out; a second diazotization product is subject to a reaction with N,N-diethyl-m-aminophenol, such that a second coupling product is obtained; the first coupling product is subject to a complexation reaction with a chromising reagent, such that a first complexation product is obtained; the first complexation product is mixed with the second coupling product, such that a second complexation reaction is carried out, and the asymmetric 1:2 blue dye represented by a formula I is obtained. According to the preparation method provided by the invention, butanone is used as a protecting agent for the diazotization product in the first coupling reaction, such that the diazotization product is more stable, the reaction between the diazotization product and 1-naphthol-5-sulfonic acid is more sufficient, the yield of the first coupling product is improved, and COD content in sewage is reduced. The formula I is presented below.
Owner:QINGDAO DOUBLE PEACH SPECIALTY CHEM GRP
Light-shielding type medical PVC (Polyvinyl Chloride) infusion tube composition and preparation method of light-shielding type medical PVC infusion tube composition
ActiveCN106700311AGood light shielding effectHigh transparencyPlasticizerHindered amine light stabilizers
The invention provides a light-shielding type medical PVC (Polyvinyl Chloride) infusion tube composition and a preparation method of the light-shielding type medical PVC infusion tube composition and belongs to the technical field of PVC infusion tube compositions. The light-shielding type medical PVC infusion tube composition is prepared from the following raw materials in parts by weight: 100 parts of polyvinyl chloride resin, 1.0 to 3.0 parts of a thermal stabilizer, 40 to 60 parts of a plasticizer, 1.5 to 4 parts of a compound light stabilizer, 0.01 to 0.025 part of titanium dioxide and 0.1 to 0.2 part of a lubricant; the compound light stabilizer is prepared by mixing a hindered amine light stabilizer, phenyl salicytate, benzotriazoles and 4-(1-naphthylazo)-1-naphthol. The light-shielding type medical PVC infusion tube composition provided by the invention has the advantages of less light capable of penetrating through a waveband of 290nm to 450nm, high transparency and no toxin.
Owner:CHINA PETROLEUM & CHEM CORP
Red reactive dye for fur and preparation thereof
InactiveCN101481528AImprove responseImprove solubilityReactive dyesDyeing processSulfonateSolubility
Owner:TIANJIN DEK CHEM
Naphthalene sulfonating method for coproducing 1-naphthol and 2-naphthol
InactiveCN108530271AIncrease productionHigh purityOrganic compound preparationSulfonic acids salts preparationDistillationSulfite salt
The invention discloses a naphthalene sulfonating method for coproducing 1-naphthol and 2-naphthol. The naphthalene sulfonating method comprises the following steps: directly neutralizing after carrying out sulfonating reaction: adding a sodium sulfite solution of which the concentration is 12 percent into a neutralizing kettle, then adding a naphthalene sulfonic acid molten material, reacting byregulating PH (Potential of Hydrogen) to be 1 to 2, heating the solution to be 105 DEG C, keeping warm for 15 minutes, and regulating the PH of the neutralizing kettle to be 7 to 8 after completing reaction; after neutralizing, sequentially carrying out naphthalene blowing, cooling crystallization, filtering, alkaline fusion, diluting, acidifying, boiling and stratifying, dewatering and drying and distillation separation, thus generating 1-naphthol and 2-naphthol. According to the naphthalene sulfonating method disclosed by the invention, a 1-naphthalene sulfonic acid isomer in a sulfonatingproduct does not need to be removed, 1-naphthol and 2-naphthol are simultaneously obtained through co-production, and the yield and the purity of 1-naphthol and 2-naphthol are effectively increased through specific production steps; a hydrolysis step is omitted, generation of wastewater during production is reduced, a production technology is simplified, and the production cost is reduced.
Owner:山西豪仑科化工有限公司
Preparation method of ultra-fine and ultra-long Au nanowire
ActiveCN107322007ASingle shapeLarge specific surface areaMaterial nanotechnologyTransportation and packagingWater bathsNanowire
The invention discloses an ultra-fast and macro-scale preparation method of an ultra-fine and ultra-long Au nanowire. The method comprises the following steps: taking ethanol and water as a mixed solvent; taking Au salt as a precursor; taking 1-naphthol as a surfactant, a reducing agent and a morphology guiding agent; and after mixing the Au salt and the 1-naphthol with the mixed solvent, carrying out water bath for heat preservation, and obtaining the ultra-fine and ultra-long Au nanowire with a length exceeding 1 micron and a particle size of about 4.5nm. Compared with a traditional preparation method, the method is simple and rapid to operate, the prepared ultra-fine and ultra-long Au nanowire is single in morphology and high in purity, and large-scale production can be realized; and the ultra-fine and ultra-long Au nanowire prepared through the method has the advantages of a large specific surface area, multiple active sites, good flexibility and the like, and has excellent electrocatalytic activity for oxygen reduction.
Owner:江苏耀兴安全玻璃有限公司
Preparation method of atovaquone
InactiveCN103570521AReduce usageReduce adverse effectsOrganic compound preparationQuinone preparation by oxidationAtovaquoneAcid catalysis
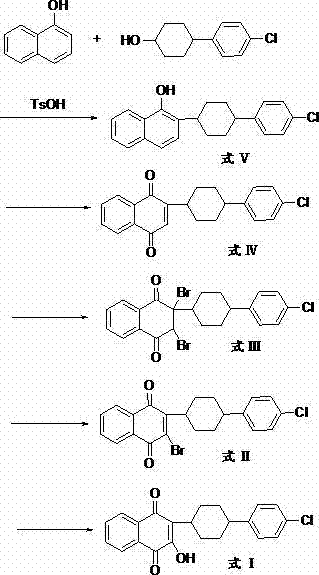
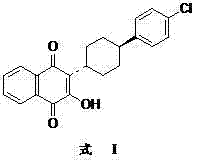
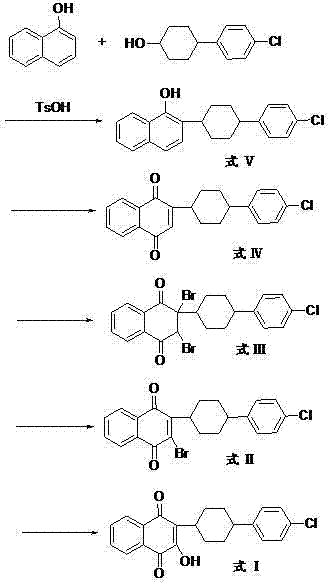
The invention discloses a preparation method of atovaquone, and belongs to the drug synthesis field. The method comprises the following steps: condensing alpha-naphthol and 4-(4-chlorophenyl)cyclohexanol under acid catalysis to obtain 2-(4-(4-chlorophenyl)cyclohexyl)-1-naphthol (formula V), oxidizing the compound represented by formula V to obtain 2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula IV), enabling the compound represented by the formula IV to react with bromine in additive reaction to obtain 2,3-dibromo-2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula III), releasing a molecule of hydrogen bromide to obtain 3-bromo-2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula II), hydrolyzing to obtain the atovaquone (formula I). Compared with the prior art, the method disclosed by the invention is simple in process, the expensive silver nitrate is prevented from using in the preparation process; and meanwhile, the yield is improved, the pollution to the environment is reduced, and the method has good popularization and application value.
Owner:SHANDONG LUKANG SHELILE PHARMA
Method for synthesizing polysubstitution 3-phenyl-1-naphthol
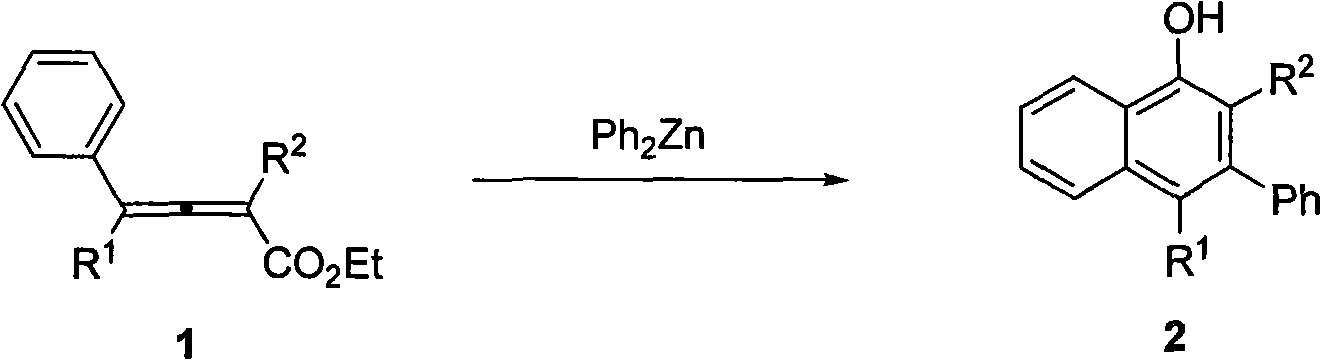
ActiveCN101619016AEasy to separate and purifyNo need to separateOrganic chemistryOrganic compound preparationSynthesis methods1-Naphthol
The invention discloses a method for synthesizing polysubstitution 3-phenyl-1-naphthol. At a temperature of 140 DEG C, dimethylbenzene is taken as a solvent, diphenylzinc agent and 2,3-linked allenoates carries out 1,4-addition reaction and intramolecular cyclization reaction to obtain a series of polysubstitution 3-phenyl-1-naphthol compounds. The reaction does not need catalysts, and the synthesis method has simple operation easily obtained raw materials and reagents, and easily separated and purified product, can introduce three substitution groups simultaneously to 1-naphthol, and is suitable for synthesizing various types of polysubstitution 3-phenyl-1-naphthol.
Owner:ZHEJIANG UNIV
Naphthol resin, epoxy resin, epoxy resin composition, and solidified products thereof
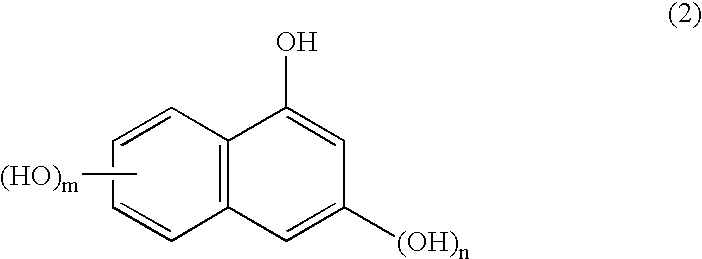
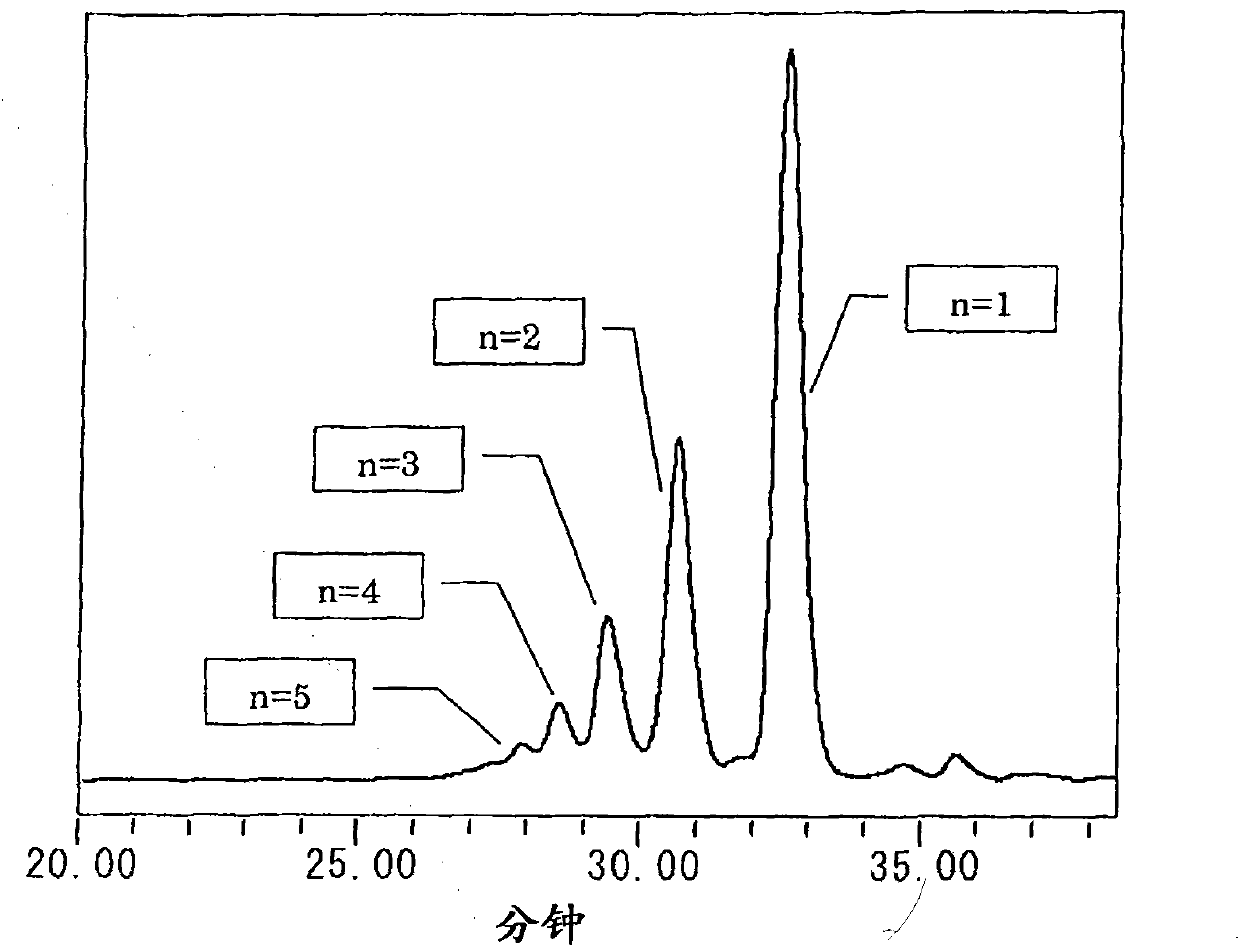
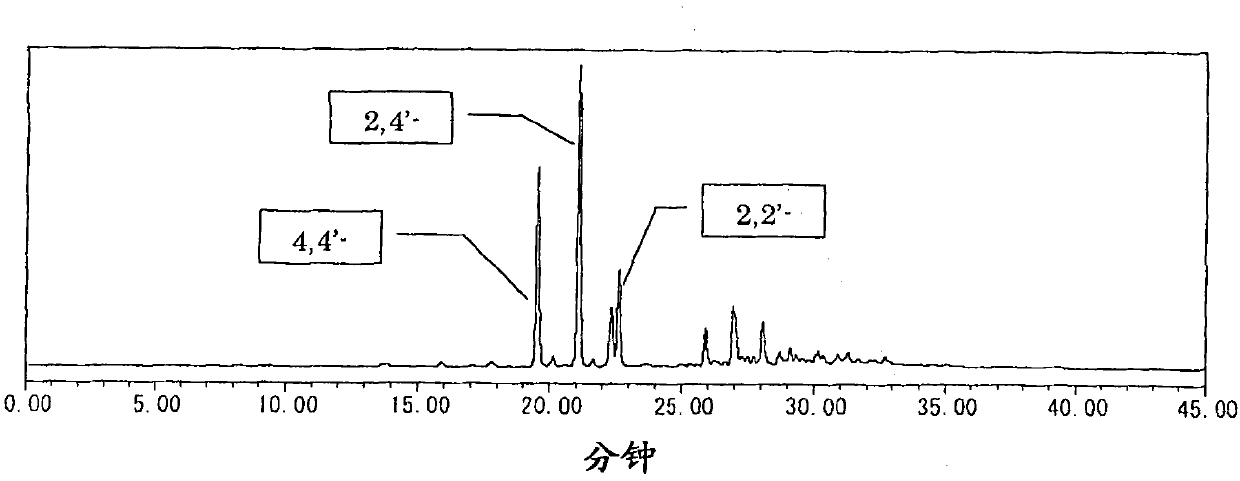
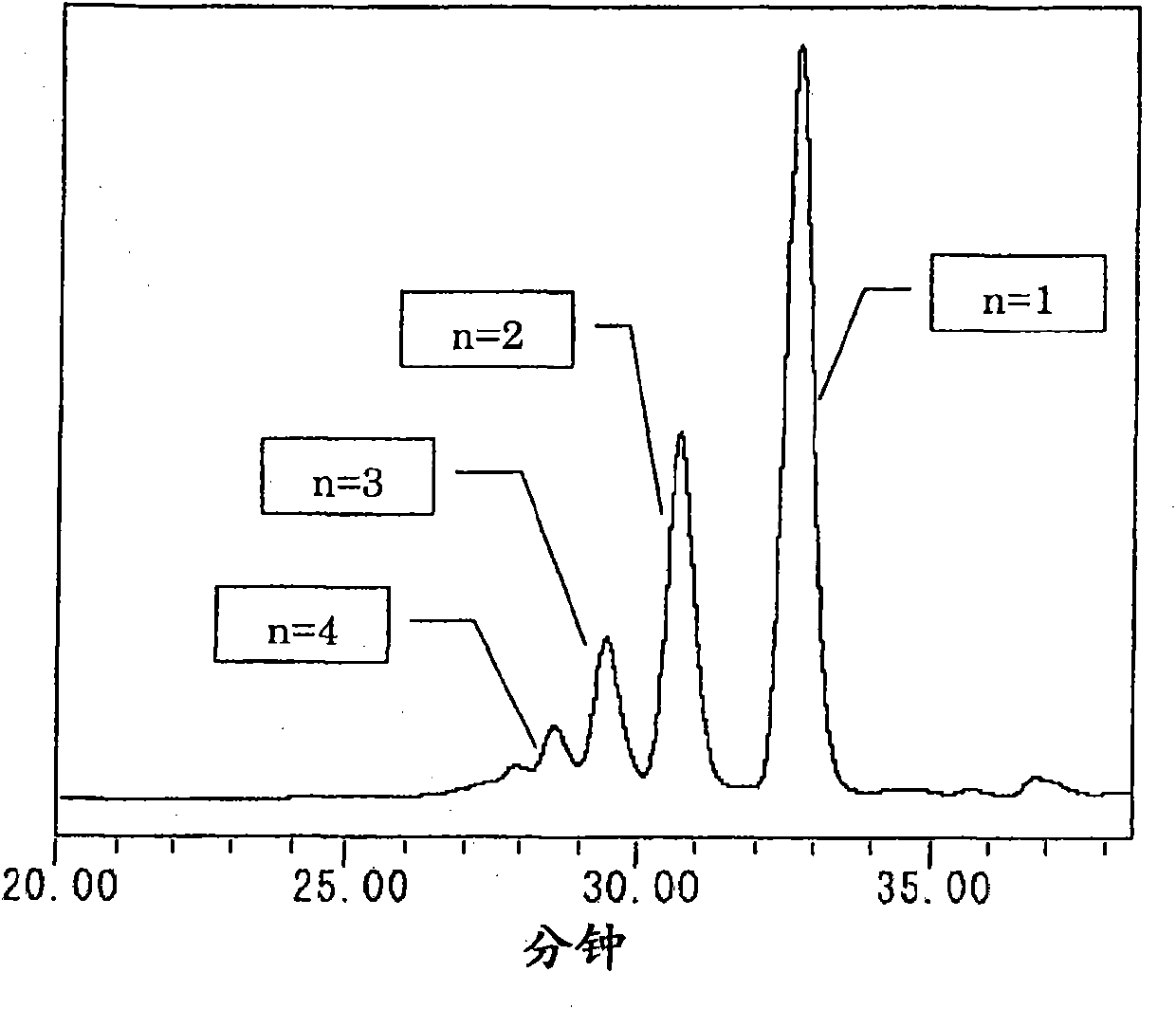
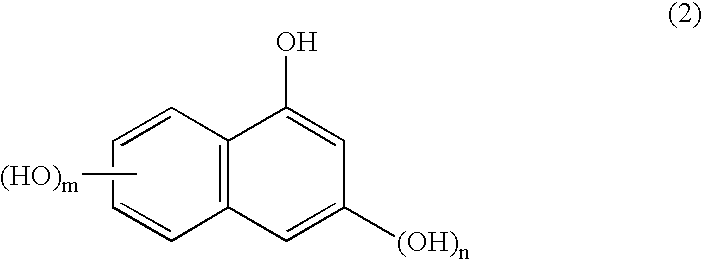
The present invention provides a novel naphthol resin and a naphthol type epoxy resin. These resins are capable of keeping an excellent forming property and a high filling property because of low viscosity and good solidifying reactivity. Solidified products of the resins are obtained by solidifying an epoxy resin composition, has characteristics of excellent fire resistance, high adhesive bonding property, good moisture-proof property and excellent heat resisting property, and has functions of lamination, forming, cast molding, adhesive bonding and the like. The naphthol resin is representedby the following general formula (1) and the epoxy resin is obtained by performing a process of epoxidation of the naphthol resin, and the present invention is characterized in that in the general formula (1), the n=1 part is 50 wt% of the whole part, displacement position of the crosslinked radical of a 1-naphthol framework in the n=1 part is 4 which is of the position of 4', the content of the isomer of 4' is between 20wt% and 60wt%, and the total content of the isomer of 4', 2 and 2' is between 40wt% and 80wt%.[the chemical formula 1].
Owner:NIPPON STEEL CHEMICALL &MATERIAL CO LTD
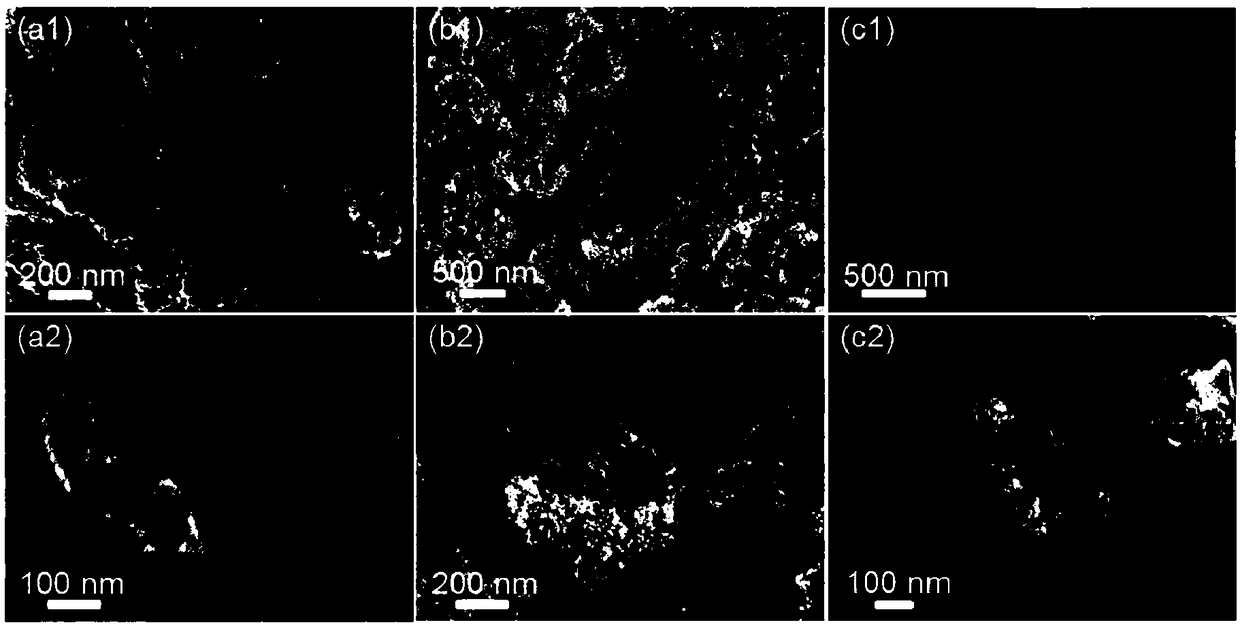
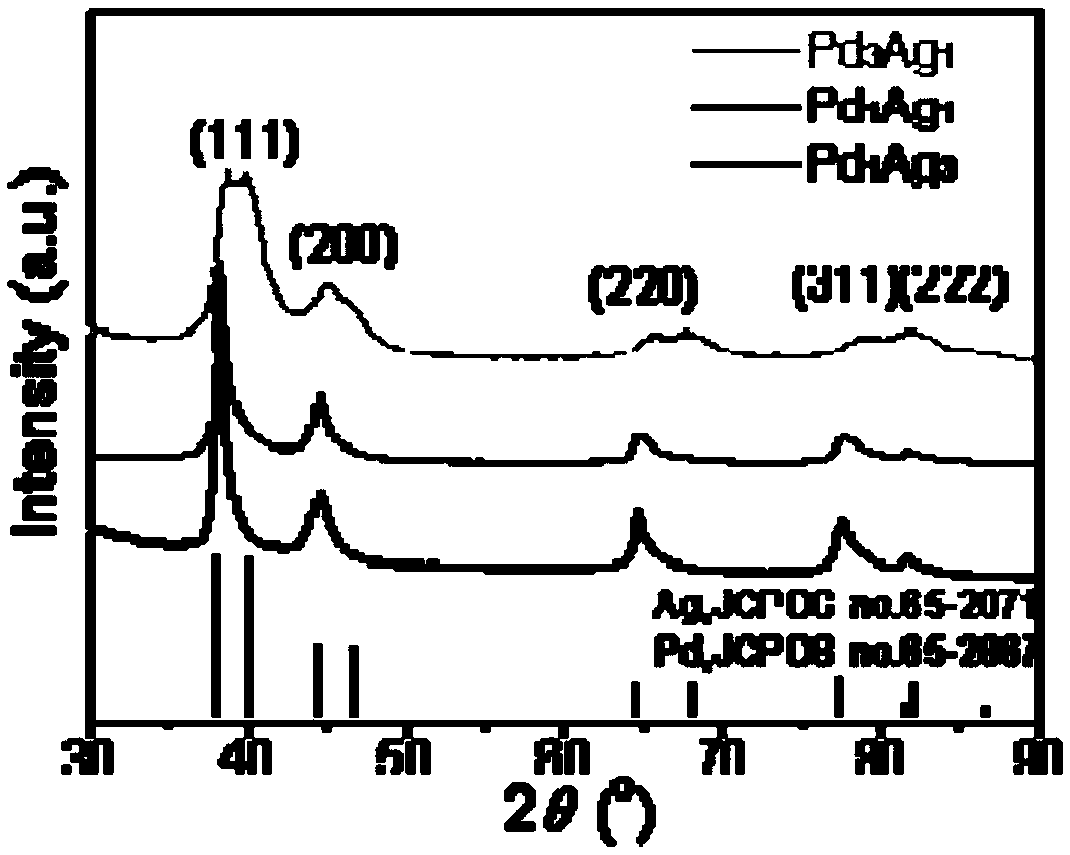
Preparation method of crown-like multistage PdAg nanodendrite, material obtained through preparation method and application
ActiveCN108666590AEasy to manufactureMild preparation conditionsMaterial nanotechnologyCell electrodes1-NaphtholSolvent
The invention discloses a preparation method of a crown-like multistage PdAg nanodendrite, a material obtained through the preparation method and an application of the material as an oxygen reductioncathode catalyst. The preparation method comprises the steps of adding a Pd-based metal precursor, an Ag-based metal precursor and 1-naphthol into a solvent, mixing evenly and then standing for reaction; and separating, washing and drying generated precipitates to obtain the crown-like multistage PdAg nanodendrite. The method disclosed by the invention is mild in condition, relatively high in yield and suitable for commercial production. The obtained crown-like multistage PdAg nanodendrite has the advantages of excellent electrocatalytic activity and stability as the oxygen reduction cathode catalyst.
Owner:NANJING NORMAL UNIVERSITY
Scale deposit inhibitor, process for its production, polymerizer whose inside wall is covered with the inhibitor, and process for production of vinylic polymers by the use of the polymerizer
InactiveUS20040077804A1Preventing adhesion of scaleAvoid stickingGaseous chemical processesLiquid-gas reaction of thin-film typeOrtho positionPhenyl group
A scale adhesion preventing agent for use in a polymerization reactor, consisting essentially of a condensate of a phenol represented by the following formula (1), a 1-naphthol and an aldehyde, which is soluble in an alkaline aqueous solution: wherein R<1 >and R<2 >are the same or different and each a hydroxyl group, alkyl group having 1 to 8 carbon atoms, halogen atom, hydrogen atom, alkoxycarbonyl group whose alkyl moiety has 1 to 8 carbon atoms or phenyl group, with the proviso that a hydrogen atom is existent at at least two of ortho-positions and para-positions with respect to the hydroxyl group. When a vinyl-based monomer is to be polymerized using a polymerization reactor having a film of this scale adhesion preventing agent on the inner wall, the vinyl-based polymer can be produced by preventing the adhesion of a scale.
Owner:TOKUYAMA CORP
Method for preparing 1-naphthol by using pigment green B catalysis
ActiveCN101723808AReduce pollutionShort routeOrganic chemistryOrganic compound preparationSolvent1-Naphthol
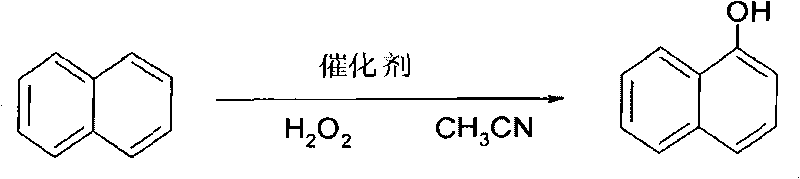
The invention provides a method for preparing 1-naphthol by using pigment green B catalysis. The method comprises the following steps: mixing naphthalene, acetonitrile solvent and pigment green B catalyst, heating the mixture to between 45 and 75 DEG C, dripping H2O2 into the mixture for 0.5 to 3 hours, and reacting the mixture for 4 to 18 hours to generate the 1-naphthol; and filtering the solution to remove the catalyst after cooling, and reclaiming the solvent to obtain a 1-naphthol product, wherein the mass ratio of the naphthalene to the H2O2 to the acetonitrile to the pigment green B is 0.26: 1.5-5: 13-30: 0.005-0.03. Compared with the prior process, the method has the advantages of cheap and easily obtained catalyst, little pollution, short line, simple operation, and easiness for industrial production.
Owner:南通新丰威机械科技有限公司
Preparation of block copolymer with quadruple responsiveness and application thereof
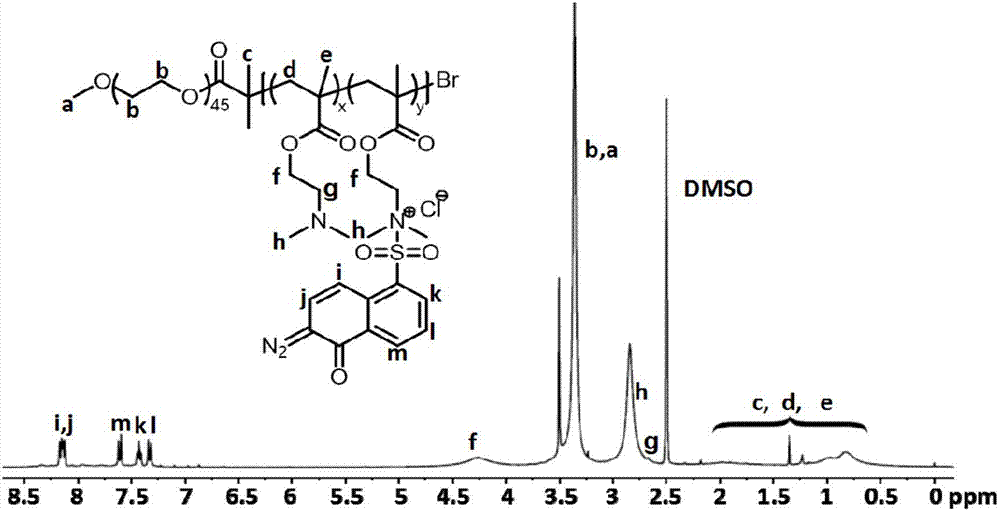
InactiveCN106893055ADrug photocleavagePharmaceutical non-active ingredientsSulfonyl chlorideUltraviolet lights
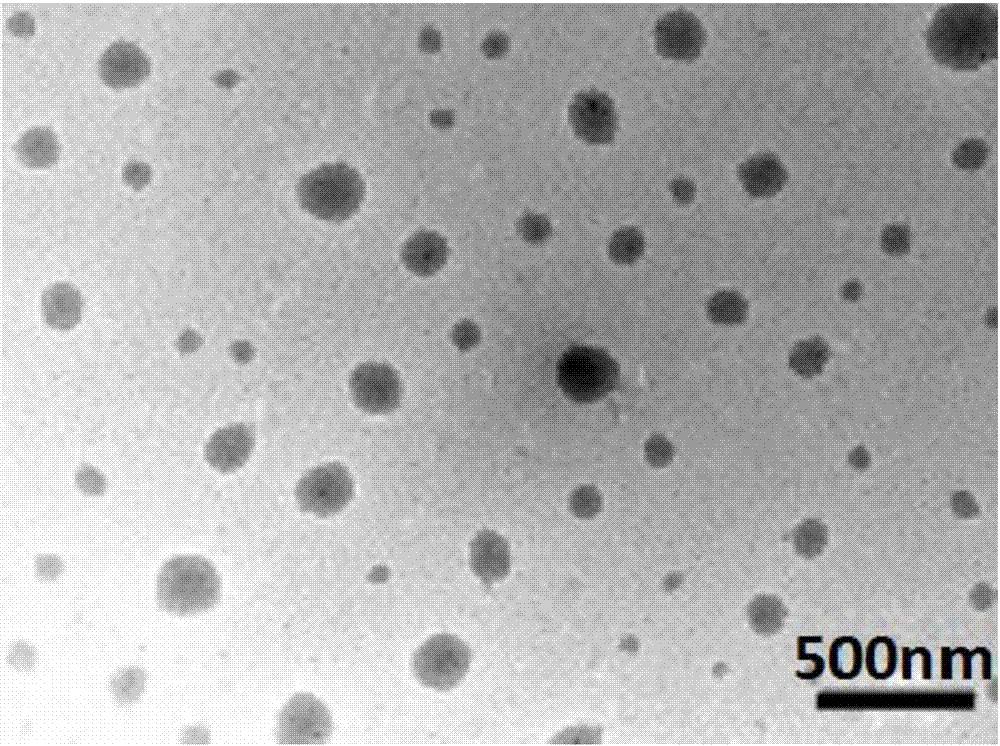
The invention provides preparation of a block copolymer micelle with quadruple responsiveness and application thereof. The preparation specifically comprises the following steps: preparing a polyethylene glycol-block-polymethylacrylic acid dimethylamino ethyl ester (PEG-b-PDMAEMA) block copolymer with a macroinitiator (mPEG-Br) and a methylacrylic acid dimethylamino ethyl ester monomer by atom transfer radical polymerization; grafting 2-diazo-1-naphthol-5-sulfonyl chloride on the polyethylene glycol-block-polymethylacrylic acid dimethylamino ethyl ester (PEG-b-PDMAEMA) block copolymer by a quaternization reaction, so as to obtain the amphiphilic block copolymer with quadruple responsiveness of near-infrared light, ultraviolet light, pH and temperature. The polymer can be self-assembled in water to form nano-particles capable of wrapping hydrophobic micromolecules such as coumarin 102; due to single or synergistic stimulation of near-infrared light 808nm, ultraviolet light 365nm, pH and temperature, the appearance of the micelle is changed, so that loaded micromolecules are released. The micelle provided by the invention has quadruple responsiveness of near-infrared light, ultraviolet light, pH and temperature and has wide application prospect in the aspect of controlling drug release.
Owner:UNIV OF SCI & TECH BEIJING
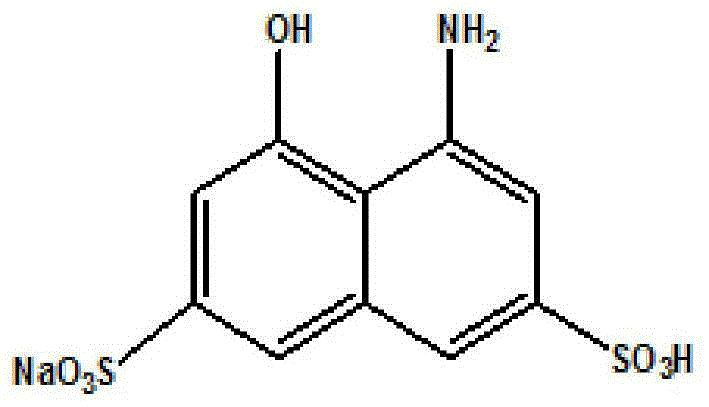
Method for synthesis of monosodium 8-amino-1-naphthol-3,6-disulfonate through hydrogenation catalysis and catalyst
InactiveCN105268450AEasy to makeEffective recoverySulfonic acids salts preparationMetal/metal-oxides/metal-hydroxide catalystsHydrogenation catalysisNaphthol AS
The invention discloses a method for synthesis of monosodium 8-amino-1-naphthol-3,6-disulfonate through hydrogenation catalysis and a catalyst. The catalyst is an active-carbon-loaded nickel molybdenum iron three-way catalyst. The method is as follows: a nitro T ammonium salt reaction liquid is employed as a reaction raw material, pH value adjustment pretreatment of the reaction raw material is carried out, then hydrogenation reduction is carried out by the active-carbon-loaded nickel molybdenum iron three-way catalyst, then alkali fusion and acidification are carried out, and monosodium 8-amino-1-naphthol-3,6-disulfonate is prepared. Preparation for the catalyst is simple, he nitro T ammonium salts can be reduced to amino T ammonium salts effectively, and the problems of catalyst poisoning, rapid inactivation and low recovery frequency and the like in the hydrogenation reduction process can be solved. The synthetic method raises the yield and the product purity of the hydrogenation catalysis technology greatly, generation of waste water and environment pollution are reduced, the technology steps are simple and safe, and the reaction is stable and easy to control.
Owner:甘肃悦美达实业有限公司
Method for preparing 1-naphthol phosphine oxide ligand
ActiveCN110183488ASimple stepsMild reaction conditionsGroup 5/15 element organic compoundsOrganic solventSulfur
The invention discloses a method for preparing a 1-naphthol phosphine oxide ligand. The method comprises the following steps: uniformly mixing a sulfur ylide compound, a phenylethyny phosphine oxide derivative, a catalyst, a Lewis acid and an organic solvent, and reacting in nitrogen at a temperature of 60-100 DEG C for 6-16 hours, thereby obtaining the finished 1-naphthol phosphine oxide ligand of a structural formula as shown in the specification. According to the method disclosed by the invention, the 1-naphthol phosphine oxide ligand that is difficultly prepared in the conventional synthetic method can be obtained, the step of the method is simple, and the 1-naphthol phosphine oxide ligand can be constructed by one step only. Moreover, the method is mild in reaction condition, excellent in substrate adaptability and high in yield, and can synthesize the 1-naphthol phosphine oxide ligand of multiple different substituent groups.
Owner:FOSHAN UNIVERSITY
Preparation method of timed-released bird repellents special for electric power and high-speed rails
ActiveCN103858878AVolatile release speed is adjustableOptimal Control StructureBiocidePest repellentsCross-linkElectric power system
The invention discloses a preparation method of timed-released bird repellents special for electric power and high-speed rails. The preparation method comprises the following steps: firstly, dissolving musk-T, cinnamamide, 1-naphthol, bisphenol A, a preservative and an antifreeze agent into N-alkyl substituted acrylamide at certain weight parts, mixing and stirring to obtain uniform dispersion liquid; and secondly, adding a cross-linking agent and an initiator into the dispersion liquid in the step I, standing for polymerization cross-linking reaction for 0.5-24h at 0-60 DEG C. The low-toxicity bird repellent composition is in-situ embedded and uniformly implanted into a gel system in a polymerization cross-linking reaction process of the N-alkyl substituted acrylamide, and the preparation method solves the technical problems that the existing universal bird repellents have high cost, low efficiency and poor weather resistance.
Owner:STATE GRID CORP OF CHINA +2
1-(2-methoxypheny1)-4-[3-(naphthalene-1-oxy)-2-hydroxypropyl] piperazine optical isomer and salt thereof, preparation method and application
InactiveCN101628899AHigh selectivityGood inhibitory effectOrganic active ingredientsOrganic chemistryEpoxySide effect
The invention relates to an optical isomer R or S of 1-(2-methoxypheny1)-4-[3-(naphthalene-1-oxy)-2-hydroxypropyl] piperazine expressed by the following formula (1) or formula (2), and also relates to a preparation method thereof. The optical isomer is characterized in that the optical isomer is obtained by the reaction of 1-(2-methoxypheny1) piperazine and intermediate which is generated by the reaction of R- or S-p-toluenesulfonyl glycidyl ester and 1-naphthol, or obtained by the reaction of 1-(2-methoxypheny1) piperazine and intermediate which is generated by the reaction of racemic epoxy chloropropane and 1-naphthol. In addition, the invention also relates to application of the optical isomer R or S and physiologically acceptable inorganic salt or organic salt thereof. Because the optical isomer R and S are selective alpha1 epinephrine receptor antagonists which have obvious inhibiting effects on benign prostatic hyperplasia and hypertension and have higher selectivity and less toxic and side effects compared with the prior alpha1 receptor antagonists used in clinic, the optical isomer R and S can be used for preparing medicaments for treating the benign prostatic hyperplasia and the hypertension.
Owner:广州医学院
Scale deposit inhibitor, process for its production, polymerizer whose inside wall is covered with the inhibitor, and process for production of vinylic polymers by the use of the polymerizer
InactiveUS6894125B2Avoid stickingGaseous chemical processesLiquid-gas reaction of thin-film typeOrtho positionPhenyl group
Owner:TOKUYAMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
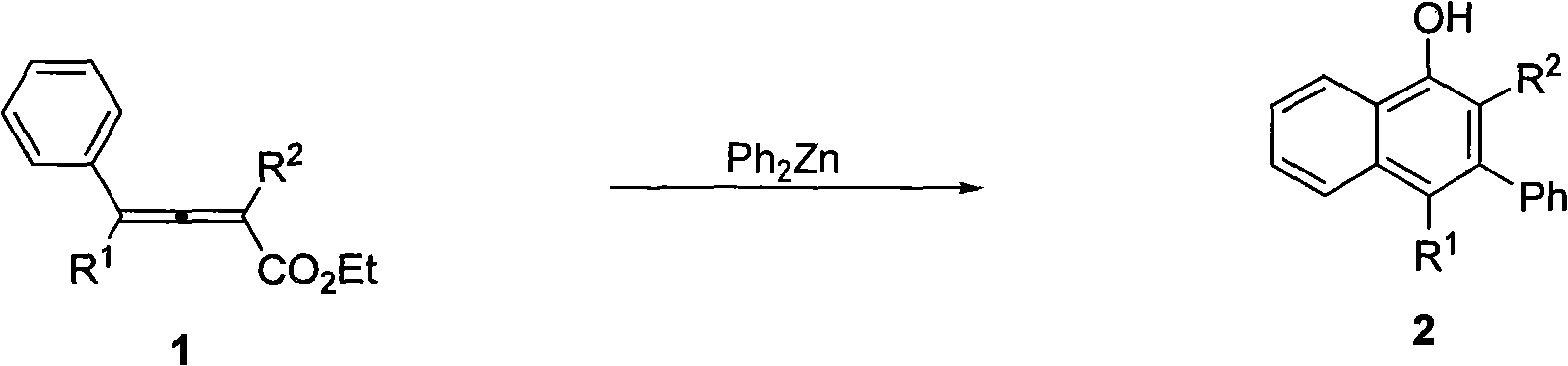
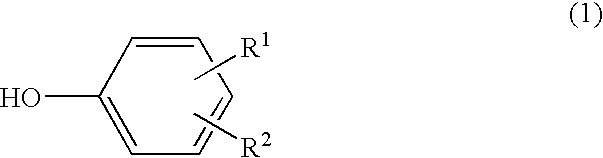
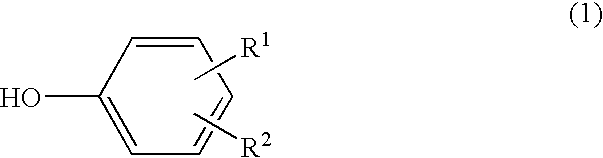
![1-(2-methoxypheny1)-4-[3-(naphthalene-1-oxy)-2-hydroxypropyl] piperazine optical isomer and salt thereof, preparation method and application 1-(2-methoxypheny1)-4-[3-(naphthalene-1-oxy)-2-hydroxypropyl] piperazine optical isomer and salt thereof, preparation method and application](https://images-eureka.patsnap.com/patent_img/33e520e9-fbf6-4d67-85f0-967019eb54e0/A2008100294270002C1.PNG)
![1-(2-methoxypheny1)-4-[3-(naphthalene-1-oxy)-2-hydroxypropyl] piperazine optical isomer and salt thereof, preparation method and application 1-(2-methoxypheny1)-4-[3-(naphthalene-1-oxy)-2-hydroxypropyl] piperazine optical isomer and salt thereof, preparation method and application](https://images-eureka.patsnap.com/patent_img/33e520e9-fbf6-4d67-85f0-967019eb54e0/A2008100294270002C2.PNG)
![1-(2-methoxypheny1)-4-[3-(naphthalene-1-oxy)-2-hydroxypropyl] piperazine optical isomer and salt thereof, preparation method and application 1-(2-methoxypheny1)-4-[3-(naphthalene-1-oxy)-2-hydroxypropyl] piperazine optical isomer and salt thereof, preparation method and application](https://images-eureka.patsnap.com/patent_img/33e520e9-fbf6-4d67-85f0-967019eb54e0/A2008100294270003C1.PNG)