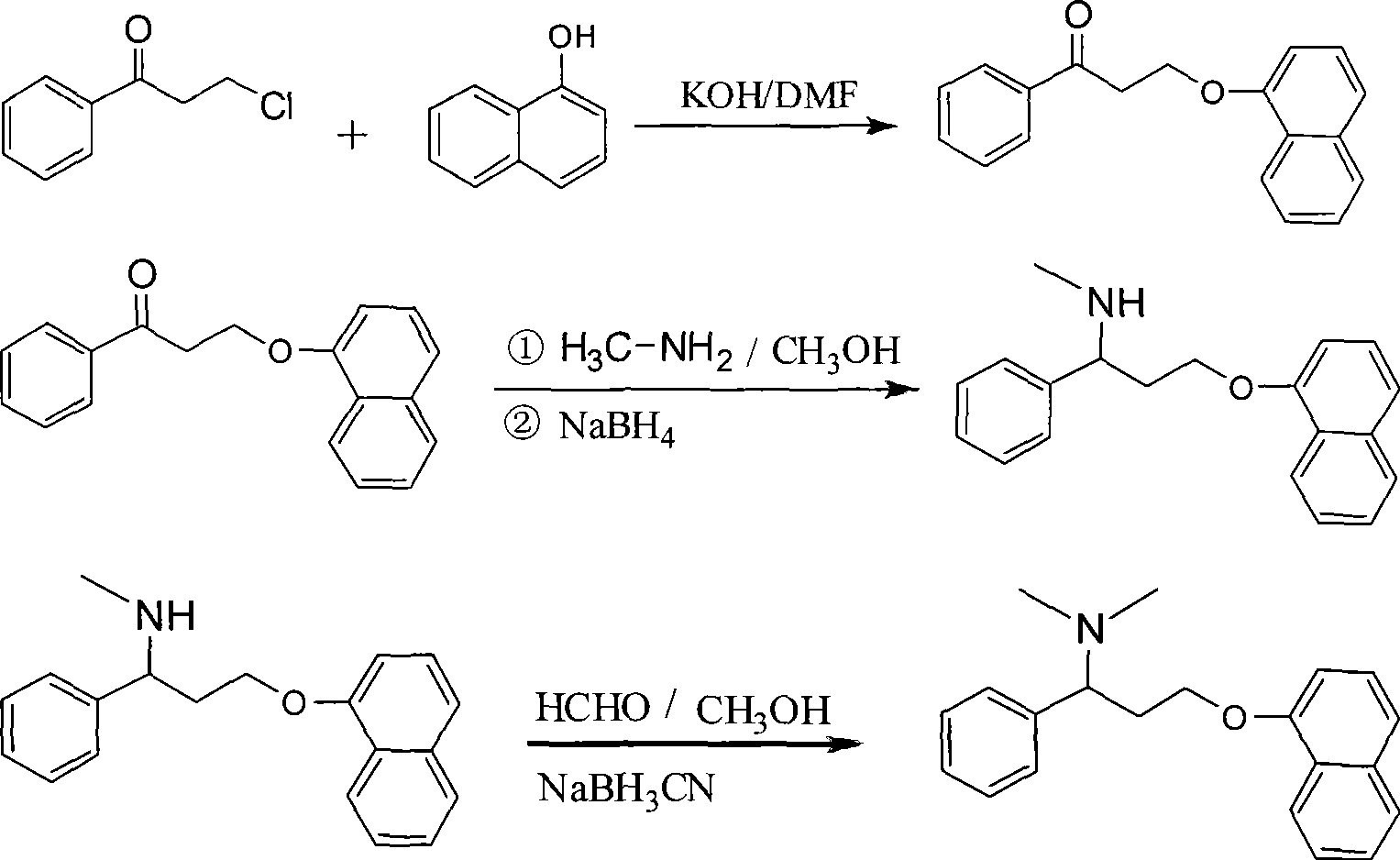
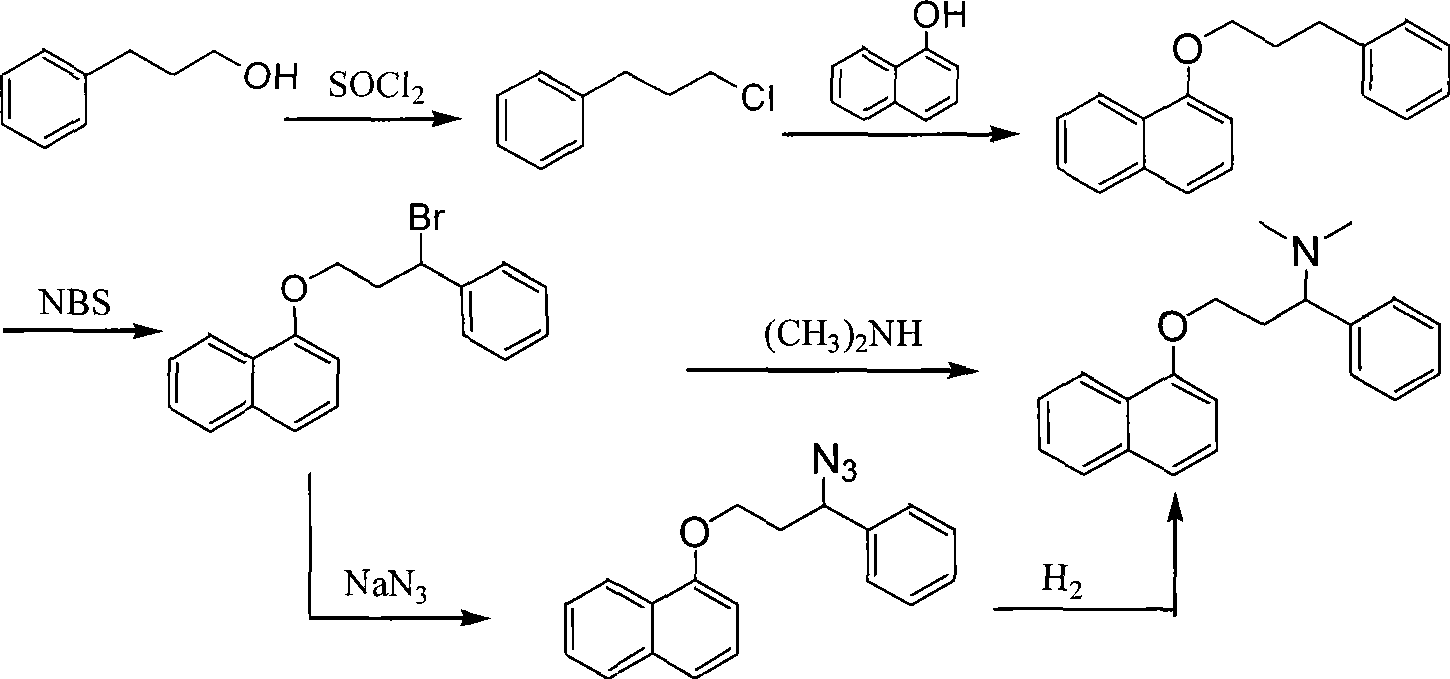
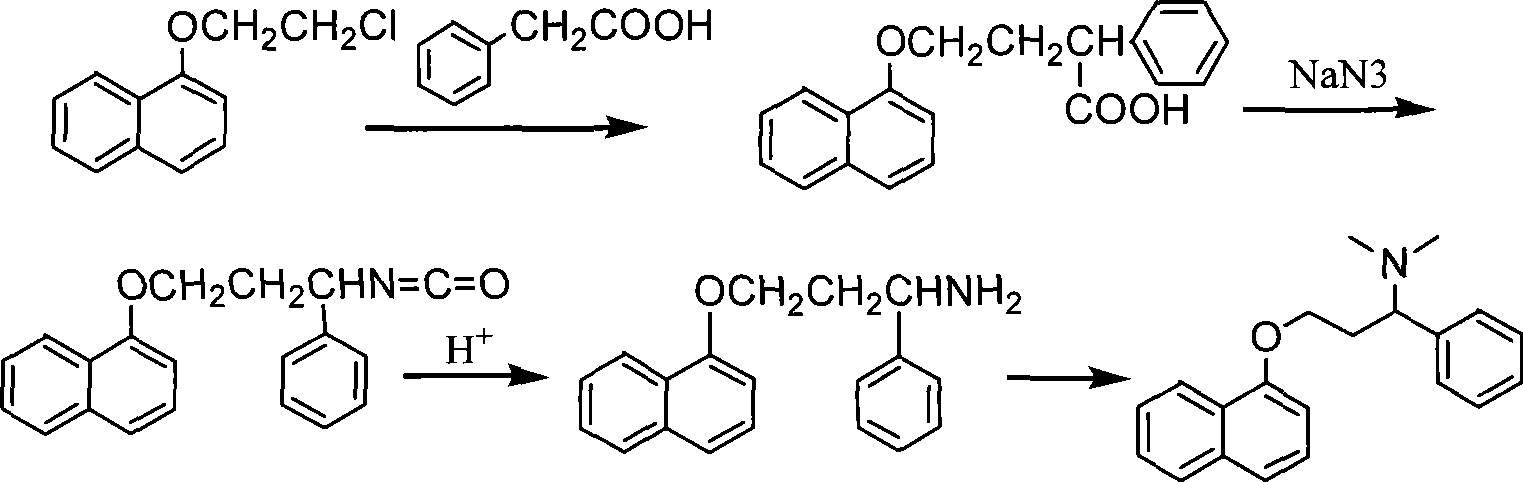
Preparation of N,N-dimethyl-1-phenyl-3-(1-naphthoxy) propanamine
A preparation process, naphthyloxy technology, applied in the field of N, can solve the problems of long synthetic route, difficult to control the source or by-product, not a synthetic method, etc., and achieve the effect of short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 3-(1-Naphthyloxy)-1-propiophenone
[0037] Dissolve 10g (0.178mol) of potassium hydroxide in N-dimethylformamide (DMF) for 150min, add 25g (0.174mol) of 1-naphthol, react at room temperature for 1h, then add 25g (0.148mol) of 3 -Chloro-1-propiophenone, reflux for 10 h, add water, extract with ethyl acetate, and concentrate to obtain 31.9 g of brown oil (unpurified, crude yield 78.1%).
Embodiment 2
[0039] N-Methyl-1-phenyl-3-(1-naphthyloxy)propylamine
[0040] 60ml of methanol, 60ml of 40% methanol solution of methylamine and 5g (0.018mol) of crude 3-(1-naphthyloxy)-1-propiophenone were sequentially added, and the mixture was refluxed for 5h. Concentrate, add 100ml of water, extract with dichloromethane, concentrate, dissolve the concentrate in 300ml of methanol, add 1g (0.027mol) NaBH at 0°C 4 , reacted for 1 h, concentrated, added water, extracted with dichloromethane, and evaporated the solvent to obtain 4.3 g of dark viscous material (unpurified, crude product yield 82.1%).
Embodiment 3
[0042] N,N-Dimethyl-1-phenyl-3-(1-naphthyloxy)propylamine
[0043] Add 4ml (0.067mol) of glacial acetic acid, 4.3g (0.015mol) of crude N-methyl-1-phenyl-3-(1-naphthyloxy)propylamine, 60ml of methanol, 7ml (0.086mol) of 37 % formaldehyde, 1.5g (0.024mol) NaBH 3 CN, react at room temperature for 1h. Concentrate, add water, extract with dichloromethane, evaporate the solvent to obtain 4 g of yellow viscous substance (yield 88.6%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com