Preparation method of joint production of alpha maphthol and beta naphthol
A technology of ethyl naphthol and methyl naphthol, which is applied in the field of co-production of methyl naphthol and methyl naphthol, can solve the problems of cumbersome operation and large three wastes, and achieve long purification steps, large discharge of three wastes, and pressure on three wastes Reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
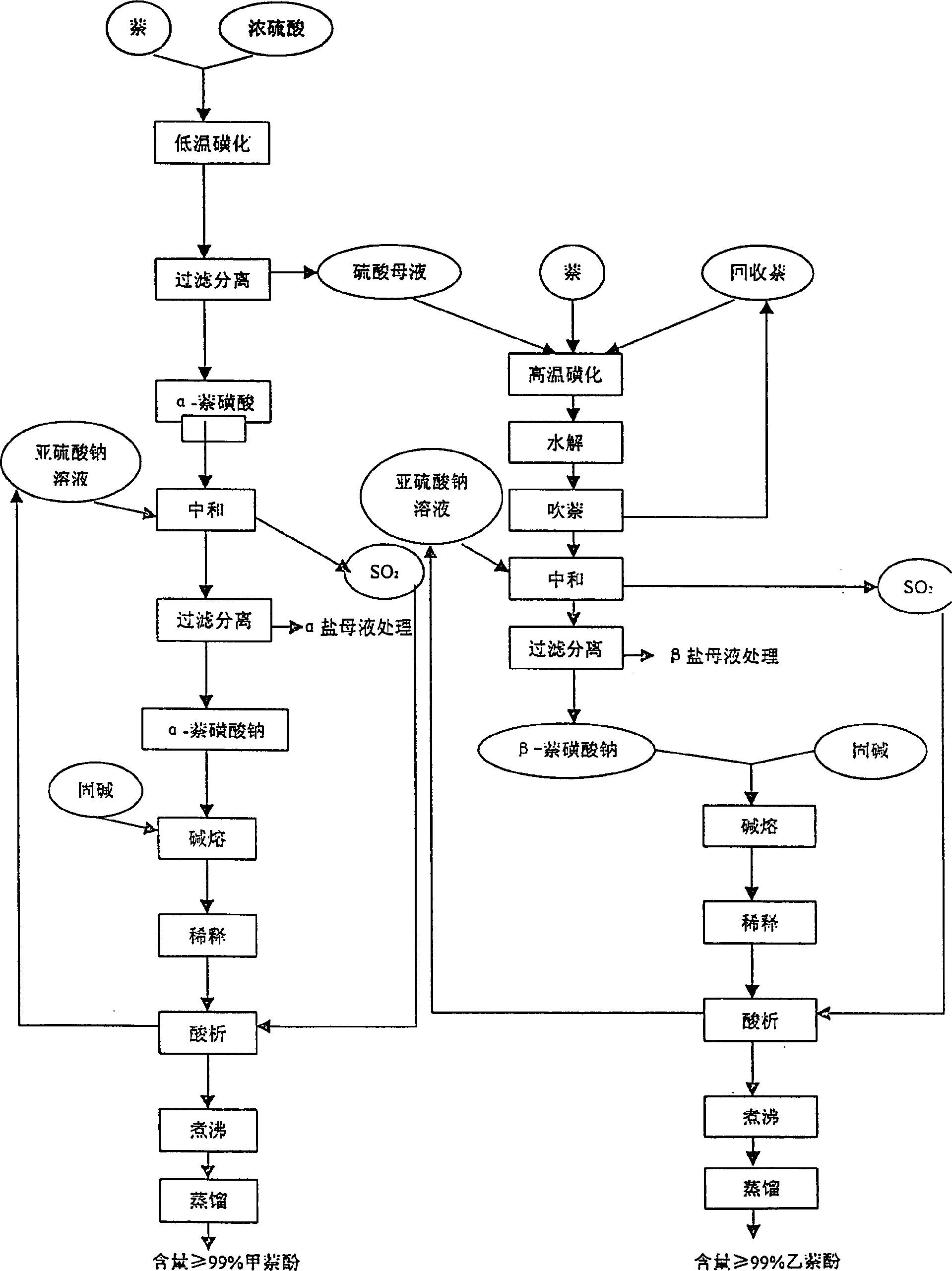
[0023] 1. Low temperature sulfonation reaction
[0024] Add 200Kg of concentrated sulfuric acid into the low-temperature sulfonation pot, control the temperature at 50-60°C, add 100Kg of refined naphthalene evenly, keep the reaction at the same temperature for 3 hours, and suction filter at 5°C to obtain acid-containing α-naphthalenesulfonate 150Kg of acid filter cake and about 150Kg of acid mother liquor.
[0025] 2. High temperature sulfonation reaction
[0026] Add 140Kg of refined naphthalene into the high-temperature sulfonation pot, control the temperature at 120-130°C, add about 150Kg of the acid mother liquor in "1. Low-temperature sulfonation reaction" dropwise, raise the temperature to 160°C and keep it warm for 3 hours to obtain β-naphthalenesulfonic acid solution.
[0027] 3. Production of high-purity methylnaphthol
[0028] 1. Neutralization
[0029] Control the temperature of 150Kg of naphthalenesulfonic acid in "1. Low-temperature sulfonation reaction" above...
Embodiment 2
[0050] 1. Low temperature sulfonation reaction
[0051] Add 150Kg of concentrated sulfuric acid (96-98%) into the low-temperature sulfonation pot, control the temperature at 70-80°C, add 100Kg of refined naphthalene evenly, keep the reaction at the same temperature for 5 hours, and suction filter at 5°C to obtain 150Kg of wet α-naphthalenesulfonic acid and about 100Kg of acid mother liquor.
[0052] 2. High temperature sulfonation reaction
[0053] Add 70Kg of refined naphthalene into the high-temperature sulfonation pot, control the temperature at 120-130°C, add about 100Kg of the acid mother liquor in "1. Low-temperature sulfonation reaction" dropwise, raise the temperature to 160°C and keep it warm for 4 hours to obtain β-naphthalenesulfonic acid solution.
[0054] 3. Production of high-purity methylnaphthol
[0055] 1. Neutralization
[0056] Control the temperature of 150Kg of naphthalenesulfonic acid in "1. Low-temperature sulfonation reaction" above 95°C, neutralize...
Embodiment 3
[0077] 1. Low temperature sulfonation reaction
[0078] Put 800Kg of concentrated sulfuric acid (96-98%) into the low-temperature sulfonation pot, control the temperature at 20-25°C, add 100Kg of refined naphthalene evenly, keep the reaction at the same temperature for 2 hours, and suction filter at 5°C to obtain Wet α-naphthalenesulfonic acid 180Kg and acid mother liquor are about 720Kg.
[0079] 2. High temperature sulfonation reaction
[0080] Add 650Kg of refined naphthalene into the high-temperature sulfonation pot, control the temperature at 120-130°C, add about 720Kg of the acid mother liquor in "1. Low-temperature sulfonation reaction" dropwise, raise the temperature to 160°C and keep it warm for 2.5 hours to obtain β-naphthalenesulfonic acid solution.
[0081] 3. Production of high-purity methylnaphthol
[0082] 1. Neutralization
[0083] Control the temperature of 180Kg naphthalenesulfonic acid in "1. Low-temperature sulfonation reaction" above 95°C, neutralize i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com