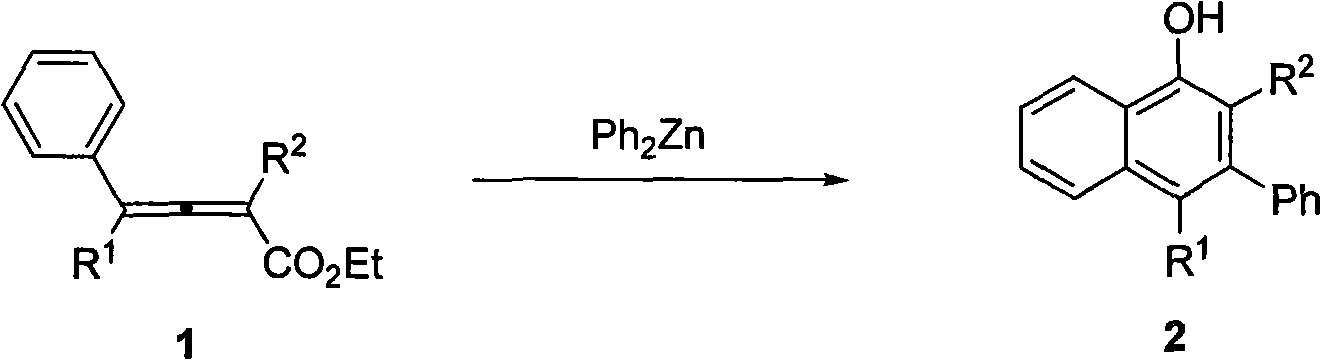
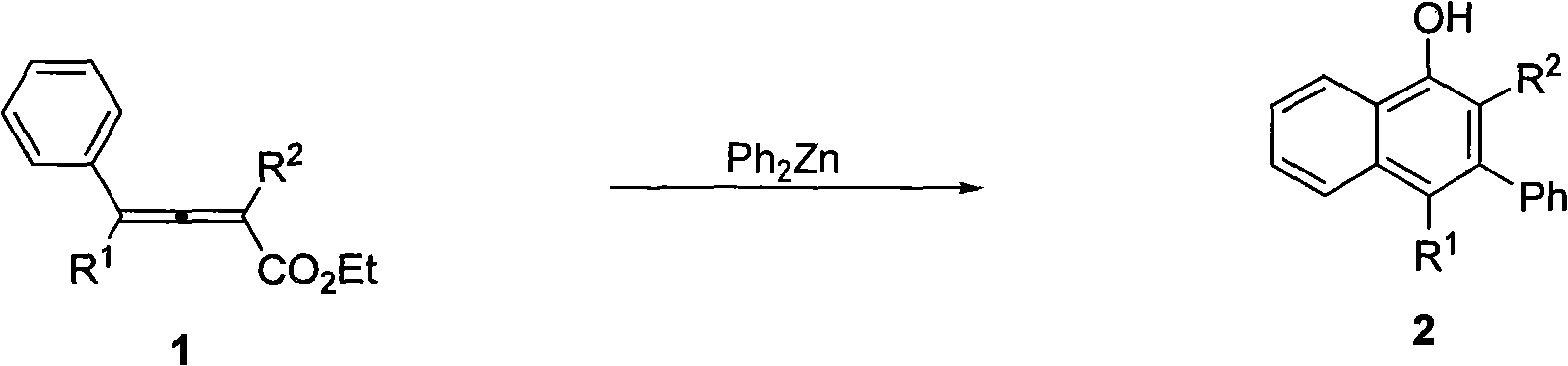Method for synthesizing polysubstitution 3-phenyl-1-naphthol
A polysubstituted, phenyl technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as difficult preparation, and achieve the effect of easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add diphenylzinc (0.1308 g, 0.6 mmol) to the reaction tube in the glove box, add xylene (3 ml) under nitrogen protection, and place in an oil bath at 140°C. Add 2-methyl-4-phenyl-2,3-butadienoic acid ethyl ester (0.0403 g, 0.2 mmol) in xylene solution (2 ml) to the reaction tube under stirring, and react at 140 degrees Celsius After 0.5 hours, add 1 ml of saturated ammonium chloride solution dropwise to quench the reaction, naturally return to room temperature and drop to zero, extract with ether, wash once with 5% hydrochloric acid, saturated sodium bicarbonate, saturated saline, and anhydrous sodium sulfate dry. Filtration, concentration, and flash column chromatography gave 0.0262 g of 2-methyl-3-phenyl-1-naphthol with a yield of 56%. The product is a white solid:
[0019] m.p.52.1-54.4℃(hexane / ethyl acetate); 1 H NMR (300MHz, CDCl 3 )δ8.17-8.09 (m, 1H), 7.79-7.72 (m, 1H), 7.52-7.32 (m, 8H), 5.24 (s, 1H), 2.27 (s, 3H); 13 C NMR (CDCl 3 , 75MHz) δ148.9, 141.7, 1...
Embodiment 2
[0021] According to the method described in Example 1, the difference is that the substrate and reagent used are: 2-methyl-4,4-diphenyl-2,3-butadienoic acid ethyl ester (0.0570 grams, 0.2 mmol) , diphenylzinc (0.1312 g, 0.6 mmol) to obtain 0.0574 g of 2-methyl-3,4-diphenyl-1-naphthol with a yield of 90%. The product is a white solid:
[0022] m.p.162.3-164.1℃(hexane / ethyl acetate); 1 H NMR (300MHz, CDCl 3 )δ8.23 (d, J = 8.7Hz, 1H), 7.56-7.41 (m, 2H), 7.40-7.28 (m, 1H), 7.25-6.95 (m, 10H), 5.27 (s, 1H), 2.14 (s, 3H); 13 C NMR (CDCl 3 , 75MHz) δ148.0, 140.6, 140.1, 139.5, 131.9, 131.6, 131.5, 130.2, 127.5, 127.4, 126.7, 126.15, 126.09, 125.7, 125.2, 123.2, 120.8, 115.3, 13.8 m / z; MS(EI) (%)311(M + +1, 25.05), 310 (M + , 100); IR(KBr, cm -1 )3550, 3444, 3057, 3028, 2924, 2856, 1592, 1497, 1442, 1374, 1289, 1212, 1160, 1097, 1027; Anal. Calcd for C 23 h 18 O: C 89.00, H 5.85, Found: C 88.97, H 5.85.
Embodiment 3
[0024] According to the method described in Example 1, the difference is that the substrate and reagent used are: ethyl 2-propyl-4,4-diphenyl-2,3-butadienoate (0.0618 g, 0.2 mmol) , diphenylzinc (0.1320 g, 0.6 mmol) to obtain 0.0618 g of 2-propyl-3,4-diphenyl-1-naphthol with a yield of 91%. The product is a white solid:
[0025] m.p.140.7-142.8℃(hexane / ethyl acetate); 1 H NMR (300MHz, CDCl 3 )δ8.24 (d, J = 8.1Hz, 1H), 7.54-7.40 (m, 2H), 7.39-7.30 (m, 1H), 7.25-6.98 (m, 10H), 5.33 (s, 1H), 2.59 -2.46(m, 2H), 1.56-1.42(m, 2H), 0.83(t, J=7.2Hz, 3H); 13 C NMR (CDCl 3 , 75MHz) δ148.0, 140.3, 140.1, 139.6, 132.0, 131.8, 131.4, 130.3, 127.3, 127.2, 126.8, 126.1, 126.0, 125.8, 125.1, 123.5, 120.9, 120.5, 29.9, 24.1; MS (E )m / z(%)339(M + +1, 26.63), 338 (M +, 100); IR(KBr, cm -1 )3581, 3417, 2959, 1588, 1498, 1445, 1380, 1203, 1160, 1100, 1029; Anal. Calcd for C 25 h 22 O: C 88.72, H 6.55, Found: C 88.79, H 6.54.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com