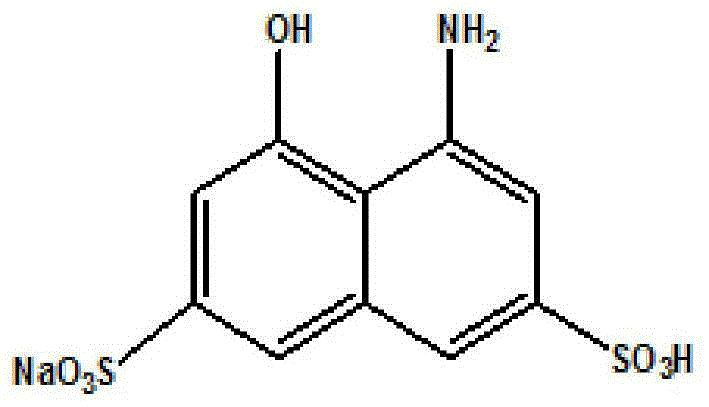
Method for synthesis of monosodium 8-amino-1-naphthol-3,6-disulfonate through hydrogenation catalysis and catalyst
A technology of hydrogenation catalysis and monosulfuric acid, applied in the field of chemistry, can solve the problems of large catalyst recycling, low catalyst recycling times, unsuitable for industrial production, etc., and achieves the effects of low cost, stable reaction and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of the catalyst used in the hydrogenation catalytic synthesis of 8-amino-1-naphthol-3,6-disulfonic acid monosodium salt: the catalyst is an activated carbon-supported three-way catalyst, which uses nickel nitrate, ammonium molybdate, nitric acid Prepare iron as a precursor to obtain a precursor solution containing nickel-molybdenum-iron active elements, and then soak activated carbon in the precursor solution for 6 to 48 hours. The element, molybdenum element, and iron element are added in a mass ratio of 1:0.2-0.5:0.1-0.2:0.01-0.2. After the nickel-molybdenum-iron active element is fully absorbed by the activated carbon, it is filtered and dried, and then placed in a high-temperature oven for curing. The high-temperature curing temperature is 200-320°C, and the high-temperature curing time is 3-12 hours. After curing, it is reduced by introducing hydrogen gas under high temperature conditions, the high temperature reduction temperature is 300-400°C, and the...
Embodiment 2
[0023] Take 1000g of the nitro-T ammonium salt reaction liquid after sulfonation, nitration, denitrification, and ammonia water neutralization as the reaction raw material, add 30% ammonia solution at room temperature, and adjust the pH of the reaction raw material to 7.5. The pretreated reaction solution is transferred into an autoclave, and an activated carbon-supported nickel-molybdenum-iron three-way catalyst is added. The catalyst is prepared by the method in Example 1, wherein the mass ratio of activated carbon, nickel element, molybdenum element, and iron element is 1:0.5:0.2:0.2, and the preparation conditions are prepared in Example 1. Activated carbon-loaded Optimal conditions for nickel-molybdenum-iron three-way catalysts. The amount of the catalyst used is 5% of the weight of the nitro T ammonium salt, the catalytic reaction temperature is 100° C., the hydrogen gas is introduced at a pressure of 1.0 MPa, and the catalytic reaction is carried out for 5 hours to obta...
Embodiment 3
[0025] Take 1000g of the nitro-T ammonium salt reaction liquid after sulfonation, nitration, denitrification, and ammonia water neutralization as the reaction raw material, add 30% ammonia solution at room temperature, and adjust the pH of the reaction raw material to 9.5. The pretreated reaction solution is transferred into an autoclave, and an activated carbon-supported nickel-molybdenum-iron three-way catalyst is added. The catalyst is prepared by the method in Example 1, wherein the mass ratio of activated carbon, nickel element, molybdenum element, and iron element is 1:0.5:0.2:0.2, and the preparation conditions are prepared in Example 1. Activated carbon-loaded Optimal conditions for nickel-molybdenum-iron three-way catalysts. The amount of the catalyst used is 20% of the weight of the nitro-T ammonium salt, the catalytic reaction temperature is 160° C., the hydrogen gas is introduced at a pressure of 5.0 MPa, and the catalytic reaction is carried out for 8 hours to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com