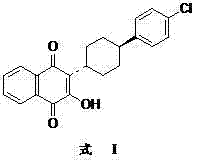
Preparation method of atovaquone
A technology of atovaquone and naphthoquinone, which is applied in the field of drug synthesis, can solve the problems of high production cost, high cost, and high price, and achieve the effects of reducing production cost, reducing adverse effects, and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
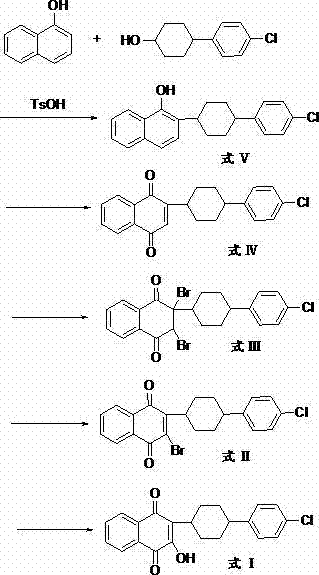
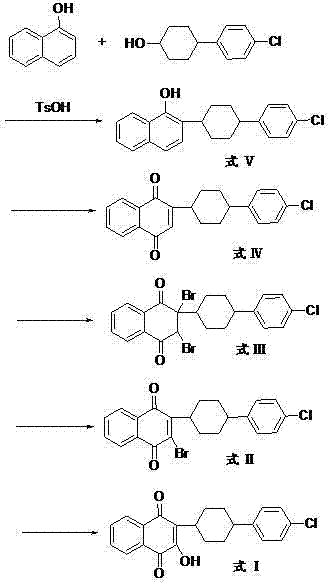
[0061] The preparation of A, 2-(4-(4-chlorophenyl) cyclohexyl)-1-naphthol (formula V)
[0062]Under the protection of nitrogen, 2.9g of 4-(4-chlorophenyl)cyclohexanol, 2g of α-naphthol, and 2.63g of p-toluenesulfonic acid were added to 30ml of toluene, and the reaction was refluxed at 140°C for 3h, and the progress of the reaction was monitored by TLC. After the reaction was complete, the organic phase was washed with water, separated to remove water, the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and crystallized to obtain 2-(4-(4-chlorophenyl)cyclohexyl)-1-naphthol.
[0063] Preparation of B, 2,3-dibromo-2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula III)
[0064] (a), the preparation of 2-(4-(4-chlorophenyl) cyclohexyl)-1,4-naphthoquinone (formula IV)
[0065] Add 2-(4-(4-chlorophenyl)cyclohexyl)-1-naphthol 2.98g to 15ml of acetonitrile, then add dropwise 4ml of concentrated hydrochloric acid (38%, v / v), 16ml o...
Embodiment 2
[0073] The preparation of A, 2-(4-(4-chlorophenyl) cyclohexyl)-1-naphthol (formula V)
[0074] Add 30ml of chlorobenzene, 2g of α-naphthol, 4.65g of 4-(4-chlorophenyl) cyclohexanol and 0.8g of p-toluenesulfonic acid in a 100ml three-necked flask, and reflux reaction for 3h. TLC monitors the reaction, and the reaction is complete. Concentrate under reduced pressure, add chloroform for extraction, wash with water, separate the organic layer, and evaporate the solvent to obtain the product 2-(4-(4-chlorophenyl)cyclohexyl)-1-naphthol.
[0075] Preparation of B, 2,3-dibromo-2-(4-(4-chlorophenyl)cyclohexyl)-1,4-naphthoquinone (formula III)
[0076] In 30ml carbon tetrachloride, add 2-(4-(4-chlorophenyl) cyclohexyl)-1-naphthol 3.36g, Br 2 1.6g, 1.2ml of 30% hydrogen peroxide solution, 0.6ml of 98% concentrated sulfuric acid, the reaction solution was refluxed for 20min, concentrated and cooled to 0°C, the solid was retained after suction filtration, an appropriate amount of water wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com