Compound tablet of dapoxetine hydrochloride and tadalafil and preparation method thereof
A technology of dapoxetine hydrochloride and tadalafil, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., and can solve the problem of affecting the release speed of sustained-release or immediate-release pellets , inaccurate drug dosage, micro-pill structure damage and other problems, to achieve good synergy, prevent micro-pill structure damage, and low crushing brittleness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
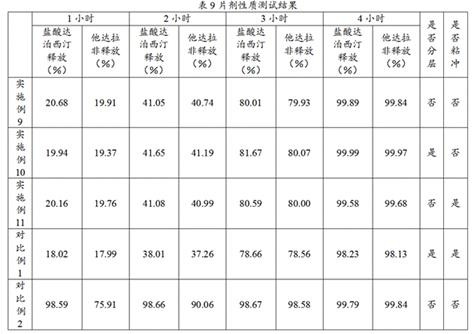
Examples
Embodiment 1
[0064] Weigh out 1 mmol γ-CD and 4 mmol K 2 CO 3 , 8 mmol NaOH or 8 mmol KOH, add 20 mL of pure water respectively, after ultrasonication to complete dissolution, use 0.8 pum water system to filter and filter to obtain the mother liquor for standby; Water methanol is placed in a closed container, heated in a water bath at 60 °C until the white turbidity is completely dissolved, and heating is continued for 20 min; immediately after taking out, add an equal volume of anhydrous methanol with a certain amount of PEG20000 dissolved, shake evenly, room temperature Let stand at 4000 rpm for 5 min, then pour off the supernatant, wash the precipitate twice with anhydrous ethanol and twice with anhydrous methanol, and dry the final solid at 40°C under vacuum overnight to obtain Nanoscale K 2 CO 3 -γ-CD-MOF, NaOH-γ-CD-MOF, KOH-γ-CD-MOF organic framework materials.
[0065] K prepared by the above method respectively 2 CO 3 - γ-CD-MOF, NaOH-γ-CD-MOF, KOH-γ-CD-MOF, γ-CD-MOF and CD a...
Embodiment 2
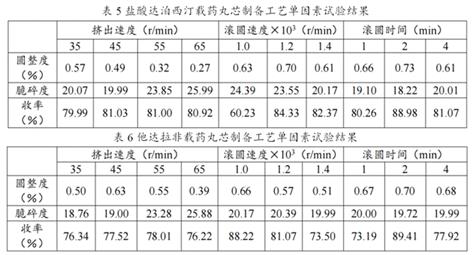
[0067] Example 2 Compression hardness test of drug-loaded matrix material
[0068] The matrix tablet was prepared by powder direct compression method. Weigh the excipients according to the prescribed amount, mix them evenly, pass through an 80-mesh sieve, mix with the sieve 3 times, dry in an oven at 40°C for 30 min, take out, add the prescribed amount of magnesium stearate, mix well, and press a 11 mm shallow arc The hardness test results are shown in Table 1.
[0069] Prescription: 18 g of drug-loaded matrix material, adding ELF Pharm and HPMC with a mass ratio of 1:1:1 K15M , Lactose powder, a total of 62 g.
[0070]
[0071] From the results in Table 1, the hardness of NaOH-γ-CD-MOF and KOH-γ-CD-MOF framework materials loaded with dapoxetine hydrochloride, and the hardness of KOH-γ-CD-MOF framework materials loaded with tadalafil Better, it can reach more than 20 kg, the compressibility is good, and there is no sticking and punching phenomenon, and it is suitable as ...
Embodiment 3
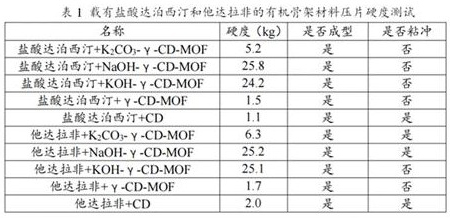
[0073] Add an appropriate amount of ultrapure aqueous solution to the centrifuge tube, add NaOH-γ-CD-MOF and KOH-γ-CD-MOF framework materials loaded with dapoxetine hydrochloride in excess, and KOH-γ- For CD-MOF skeleton material, place the centrifuge tube in an air-bath thermostatic shaker at 37°C, 100 r / min, shake well for 24 h, take the supernatant, and filter with a 0.22 μm membrane. The drug loading in the drug-loaded matrix material was tested. Precisely weigh 1 mg of each drug-loaded matrix material, dissolve them in 1 mL of ultrapure water, and filter them with a 0.22 μm filter membrane to determine the drug-loading amount. The results are shown in Table 2.
[0074]
[0075] From Table 2, it can be seen that the drug loading of NaOH-γ-CD-MOF framework materials loaded with dapoxetine hydrochloride is higher than that of KOH-γ-CD-MOF, so NaOH-γ-CD-MOF was selected as the next study. skeleton material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet weight | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com