Synthesis method of dapoxetine
A synthesis method and technology of dapoxetine, applied in the field of drug preparation, can solve the problems of difficult reaction control, increased production cost, complicated operation and the like, and achieve the effects of avoiding volatilization, saving costs and being cheap.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
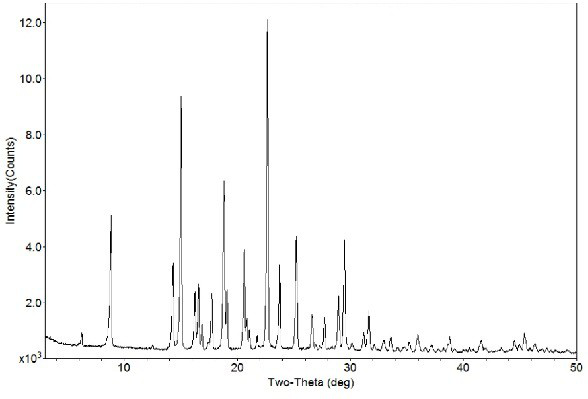
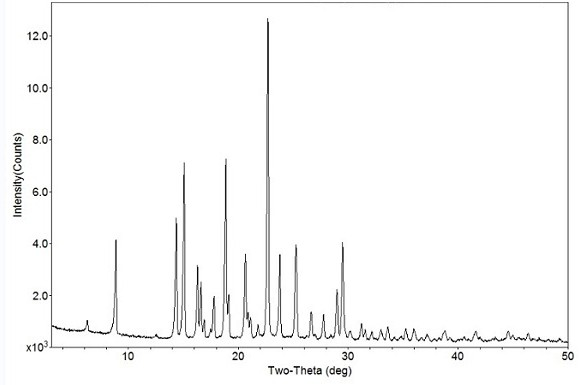
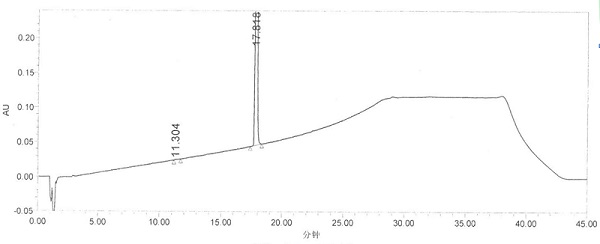
Image
Examples
preparation example Construction
[0050] The synthetic method of dapoxetine of the present invention comprises the following steps:
[0051] S1. Disperse (s)-3-amino-3-phenylpropanoic acid or its esters in a solvent, and perform a reflux reaction under the action of a reducing agent to obtain (s)-amino-3-phenylpropanol;
[0052] S2. Dissolve (s)-amino-3-phenylpropanol obtained in step S1 in formic acid aqueous solution, add paraformaldehyde and heat up to react to obtain (s)-3-dimethylamino-3-phenylpropanol ;
[0053] S3. Dissolve the (s)-3-dimethylamino-3-phenylpropanol obtained in step S2 in a solvent, and under the protection of nitrogen, add it dropwise to the alkali solution at a higher temperature of >30°C to react, and then Adding 1-fluoronaphthalene takes place Williamson ether formation reaction to obtain (s)-N,N-dimethyl-3-(1-naphthyloxy)amphetamine, ie dapoxetine.
[0054] The reaction process of the present invention is as follows:
[0055] .
[0056] The synthetic method of dapoxetine of the...
Embodiment 1
[0068] Step S1. Preparation of intermediate 1: (s)-3-amino-3-phenylpropanol.
[0069] At 0~10°C, add 20.2kg of tetrahydrofuran into a 200L reaction kettle, stir, add 3.8kg of (s)-3-amino-3-phenylpropionic acid, add 1.74kg of sodium borohydride, and keep dropping at 0~20°C Add iodine-tetrahydrofuran solution (dissolve 5.9kg iodine in 10.1kg tetrahydrofuran solution and add dropwise for 5~6h), and the dripping is completed. Raise the temperature to reflux (60~70°C) and react for 14~18h, and the central control raw materials basically react completely. Cool down to below 20°C, add 9.0kg of methanol dropwise to quench, and add dropwise for 20~30min. The organic solvent was distilled off under reduced pressure until no obvious liquid drops flowed out to obtain a white oil. Add 22.8 kg of 20% (mass concentration) sodium hydroxide solution to the concentrate to adjust the pH value to 10~13, and stir at 25~35°C for 3 hours. Add dichloromethane for extraction, extract twice, 20.0kg ...
Embodiment 2
[0079] Step S1. Preparation of intermediate 1: (s)-3-amino-3-phenylpropanol.
[0080] At 0~10℃, add 33.0kg of tetrahydrofuran into a 200L reaction kettle, stir, add 6.2kg of (s)-3-amino-3-phenylpropionic acid methyl ester, add 2.79kg of sodium borohydride, keep 0~20 Add iodine-tetrahydrofuran solution dropwise at ℃ (dissolve 9.5kg iodine in 16.4kg tetrahydrofuran solution and add dropwise for 5~6h), and dropwise complete. Raise the temperature to reflux (60~70°C) and react for 14~18h, and the central control raw materials basically react completely. Cool down to below 20°C, add 14.7kg of methanol dropwise to quench, and add dropwise for 20~30min. The organic solvent was distilled off under reduced pressure until no obvious liquid drops flowed out to obtain a white oil. Add 37.2kg of 20% sodium hydroxide (mass concentration) solution to the concentrate to adjust the pH value to 10~13, and stir at 25~35°C for 3h. Add dichloromethane for extraction, extract twice, 30kg each ti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com