Crystal and amorphous substance of dapoxetine hydrochloride and preparation method thereof
A technology of dapoxetine hydrochloride and dapoxetine, which is applied in the preparation of organic compounds, the preparation of aminohydroxy compounds, and organic chemical methods, etc., can solve the problems of unobtained product dapoxetine hydrochloride and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Reference Example A Preparation of Crystal Form Dapoxetine Hydrochloride
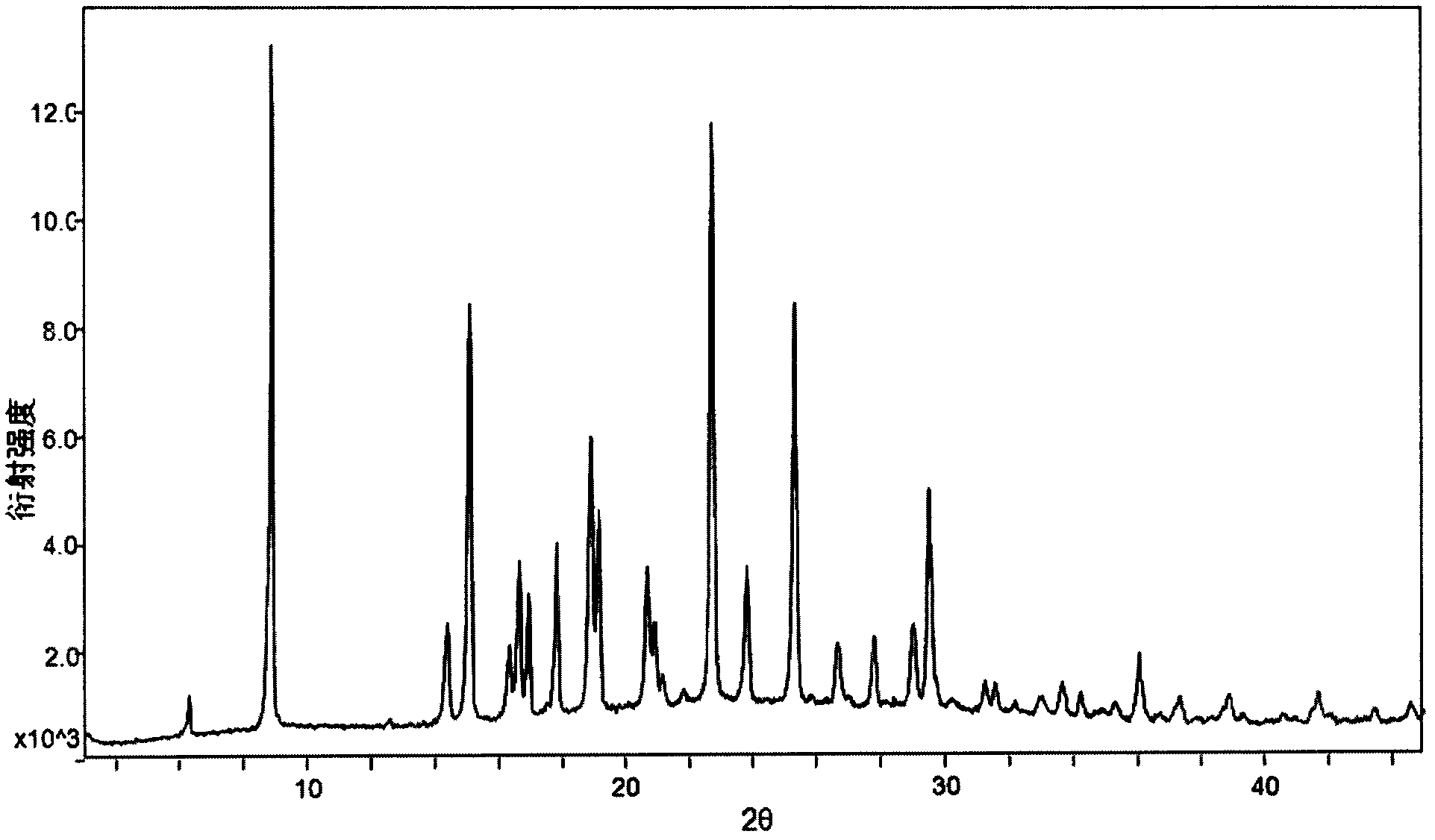
[0034] Weigh 916mg of dapoxetine into a container, add 15ml of ethyl acetate at room temperature, stir until the solid is completely dissolved, filter out insoluble impurities, then pass dry hydrogen chloride gas into it, stir for 2 hours at room temperature, and precipitate , and filtered to obtain 620 mg of white powder, which is the A crystal form of dapoxetine hydrochloride. Its XRPD pattern and DSC pattern see image 3 and Figure 4 . The 2θ value of its XRPD pattern is 6.33, 8.92, 14.42, 15.11, 16.34, 16.65, 16.95, 17.84, 18.93, 19.18, 20.70, 20.93, 21.16, 22.73, 23.82, 25.34, 26.66, 27.80, 29.03, 29.25 , 33.03, 33.67, 34.24, 36.05, 37.32, 38.88, 41.68, 43.45, 44.58, there are diffraction peaks, and the error range of the 2θ value is ±0.2; the DSC spectrum has an endothermic peak at 177±5°C.
Embodiment 1B
[0035] The preparation of embodiment 1B crystal form dapoxetine hydrochloride
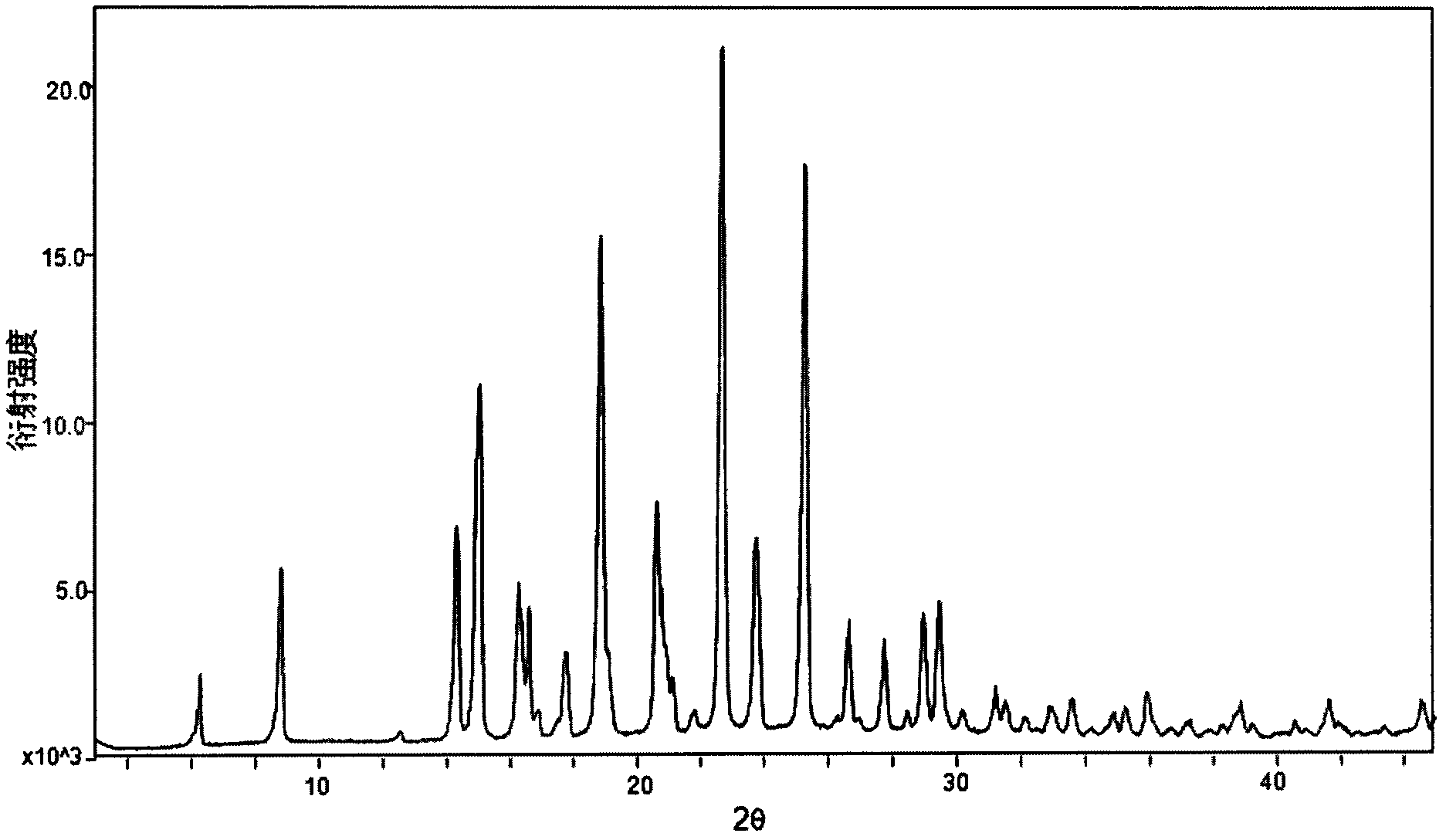
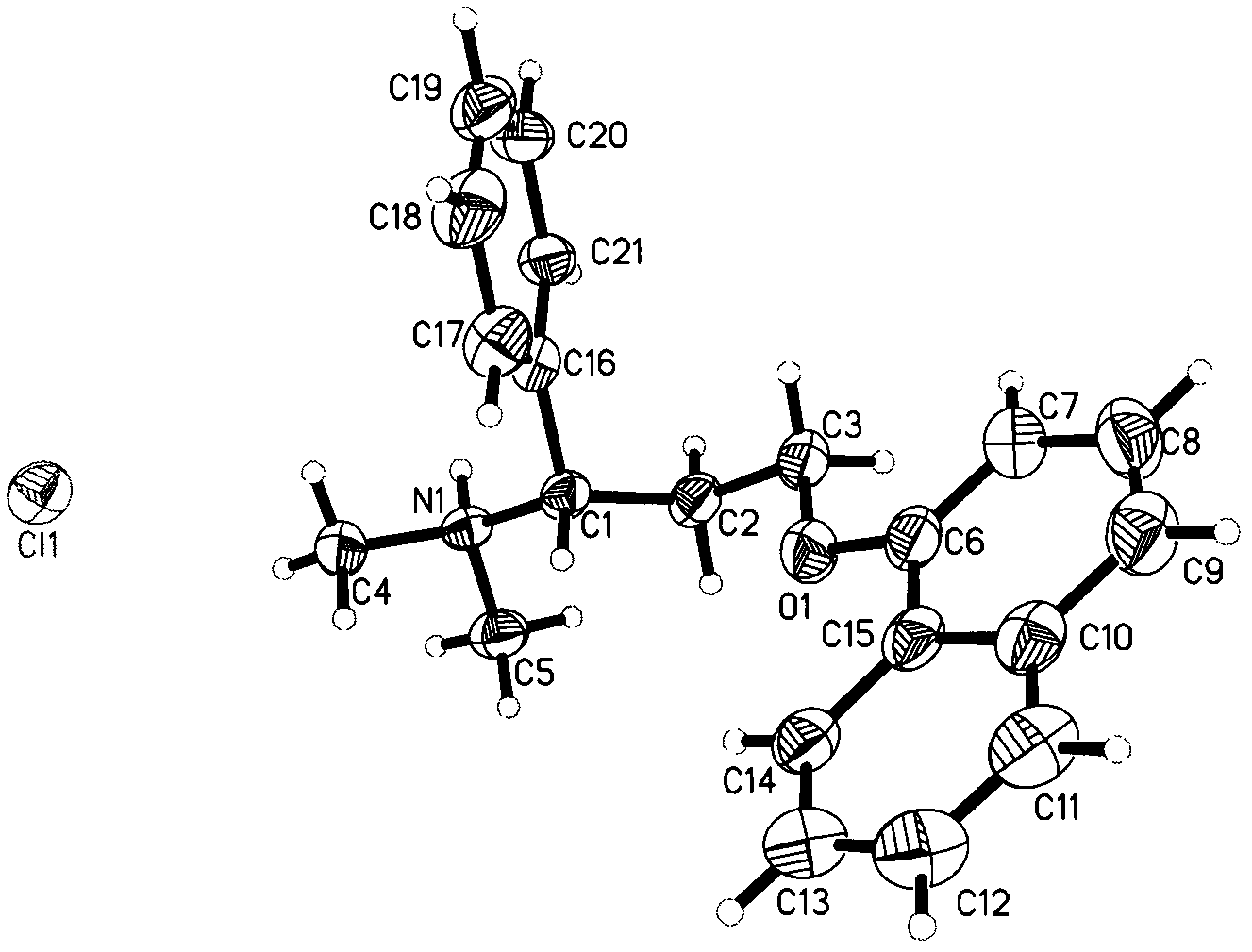
[0036] Weigh 916mg of dapoxetine into a container, add 15ml of dichloromethane, stir at room temperature until the solid is completely dissolved, filter off the insoluble matter, then slowly drop into 1.5ml of a saturated solution of hydrogen chloride in dichloromethane, stir at room temperature for 2h, there is precipitation Precipitate, lower the temperature to 5° C., filter, and vacuum-dry to obtain 800 mg of white powder, which is Dapoxetine Hydrochloride in Form B of the present invention. Its XRPD pattern and single crystal structure diagram are shown in figure 1 and figure 2 . In its XRPD pattern, the radiation source is CuKα 1 , at the diffraction angle 2θ values are 6.29, 8.84, 14.33, 15.06, 16.28, 16.38, 16.61, 16.89, 17.78, 18.87, 20.62, 20.76, 21.10, 21.82, 22.69, 23.78, 25.30, 26.66, 27.75, 28.976, , 30.19, 31.22, 31.54, 32.11, 32.96, 33.57, 34.91, 35.25, 35.92, 38.88, 41.64, 44.5...
Embodiment 2
[0037] The preparation of embodiment 2 amorphous dapoxetine hydrochloride
[0038] Dissolve 500 mg of dapoxetine hydrochloride (form B obtained in Example 1) in 20 mL of methanol, filter out the insoluble matter after complete dissolution, quickly remove the solvent by rotary evaporation, and obtain a white solid after vacuum drying, which is the amorphous compound of the present invention. Form Dapoxetine hydrochloride. Its XRPD spectrum and DSC spectrum are shown in Figure 5 and Figure 6 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com