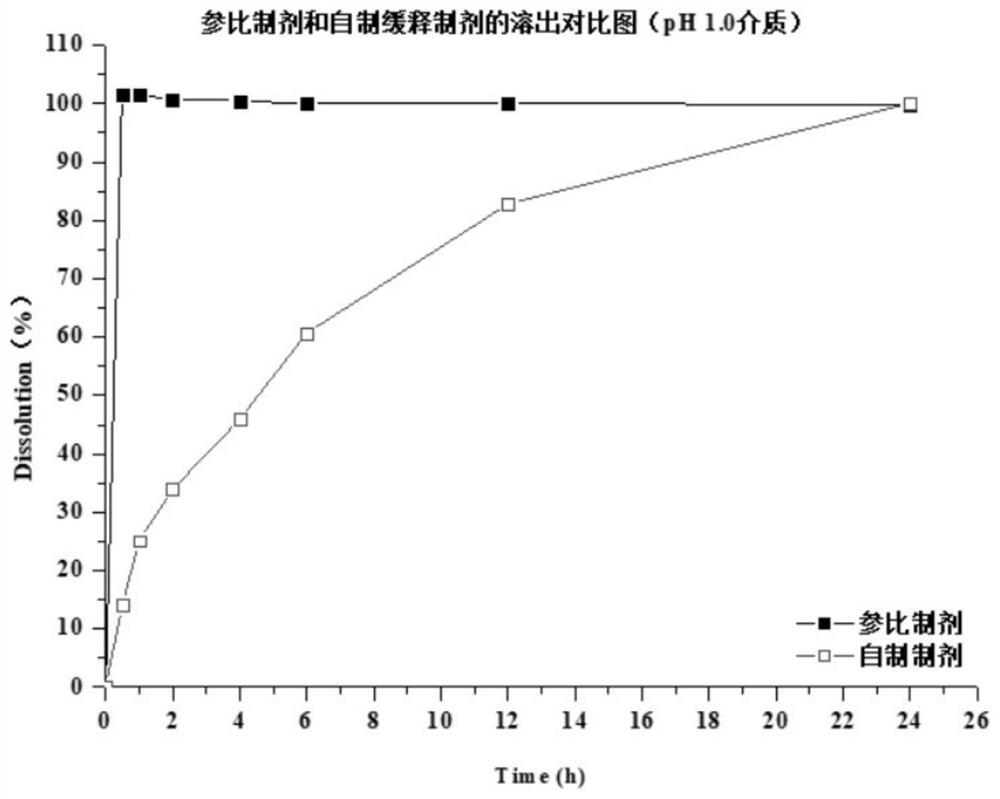
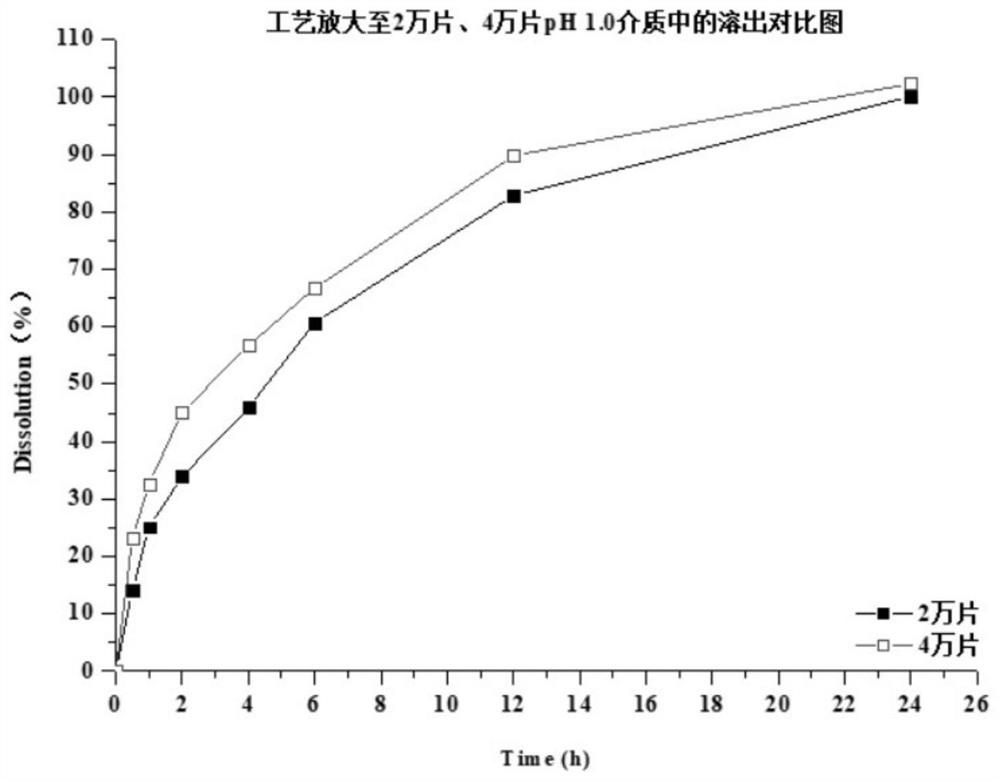
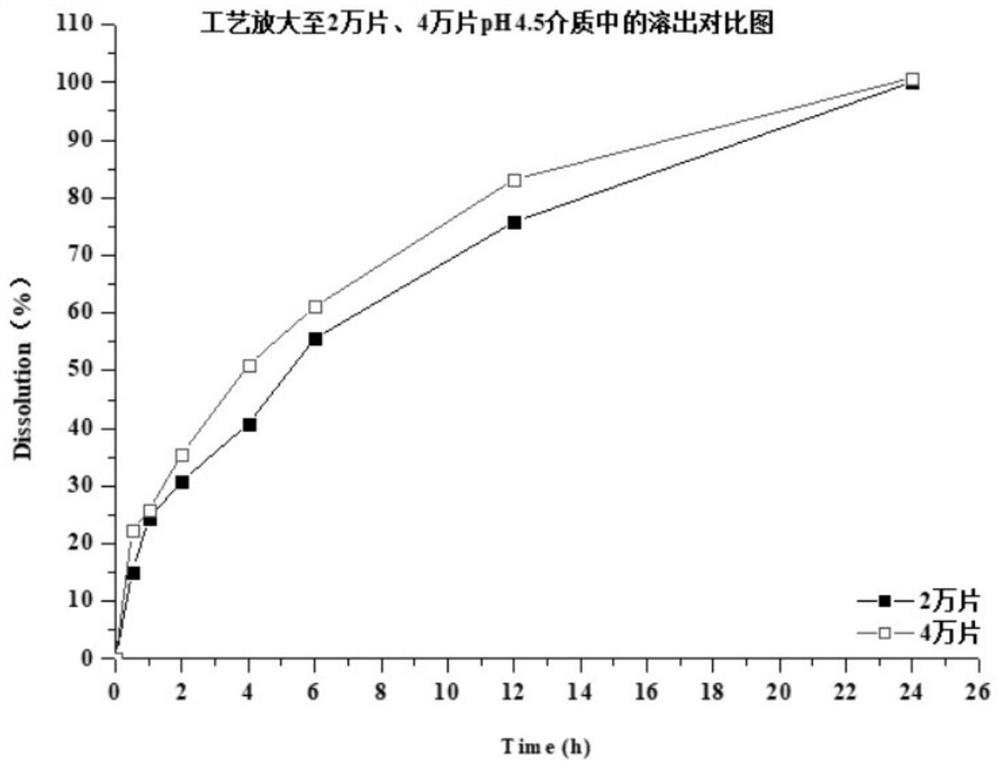
Preparation method of dapoxetine hydrochloride sustained release tablet
A technology of dapoxetine hydrochloride and sustained-release tablets, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problem of patients not receiving clinical knowledge of premature ejaculation, etc. The effect of lasting blood drug concentration, improving use safety and process stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Specification: 30mg, batch size is 1000 tablets. The prescribing information is as follows:
[0052]
[0053] The preparation process is as follows:
[0054] 1. Premixing: dapoxetine hydrochloride raw material 33.6g, croscarmellose sodium 4g, ethyl cellulose 10g, lactose 44.4g, in a wet granulator, stirring speed 300rpm, shearing speed 1800rpm, pre-mixing Mix for 7 minutes;
[0055] 2. Granulation: Dissolve 5g of ethyl cellulose in 18g of purified water to make a solution, and spray it into the premix. It is required to complete the spraying within 1 minute, and then granulate for 4 minutes;
[0056] 3. Wet granulation: Wet granulation with 20-mesh sieve in wet granulation swinging granulator;
[0057] 4. Drying: the air inlet temperature of the fluidized bed is 60°C, the material temperature is 35-45°C, and dried to a moisture content of 2.0%-3.0%;
[0058] 5. Dry granulation: dry granules pass through a 20-mesh sieve for dry granulation;
[0059] 6. Total mixi...
Embodiment 2
[0063] The prescribing information is as follows:
[0064] The batch size is 20,000 pieces.
[0065]
[0066] The preparation process is as follows:
[0067] 1. Premixing: dapoxetine hydrochloride raw material 672g, croscarmellose sodium 20g, ethyl cellulose 200g, lactose 1008g, wet granulator, stirring speed 400rpm, shearing speed 2100rpm, premixing 7min ;
[0068] 2. Granulation: Dissolve 20g of ethyl cellulose in 360g of purified water to make a solution, add it by spraying, spraying is required to be completed within 3 minutes, and then granulate for 3 minutes;
[0069] 3. Wet granulation: Wet granulation with 20-mesh sieve in wet granulation swinging granulator;
[0070] 4. Drying: the air inlet temperature of the fluidized bed is 60°C, the material temperature is 35-45°C, and dried to a moisture content of 2.0%-3.0%;
[0071] 5. Dry granulation: dry granules pass through a 20-mesh sieve for dry granulation;
[0072] 6. Total mixing: Calculate the amount of added ...
Embodiment 3
[0076] The prescribing information is as follows:
[0077] Batches are 40,000 pieces.
[0078]
[0079] The preparation process is as follows:
[0080] 1. Premixing: dapoxetine hydrochloride raw material 1344g, croscarmellose sodium 160g, ethyl cellulose 200g, lactose 2056g, wet granulator, stirring speed 400rpm, shearing speed 2100rpm, premixing 7min ;
[0081] 2. Granulation: Dissolve 120g of ethyl cellulose in 720g of purified water to make a solution, add it by spraying, spraying is required to be completed within 3 minutes, and then granulate for 3 minutes;
[0082] 3. Wet granulation: Wet granulation with 20-mesh sieve in wet granulation swinging granulator;
[0083] 4. Drying: the air inlet temperature of the fluidized bed is 60°C, the material temperature is 35-45°C, and dried to a moisture content of 2.0%-3.0%;
[0084] 5. Dry granulation: dry granules pass through a 20-mesh sieve for dry granulation;
[0085] 6. Total mixing: Calculate the amount of added sil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com