Separation and determination methods for dapoxetine hydrochloride intermediate SM1 and related impurities
A technology for dapoxetine hydrochloride and intermediates, applied in the field of analytical chemistry, can solve the problem of not separating SM1 and related impurities SM1 and related impurities, and achieve effective control, accurate measurement, and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 The positioning of dapoxetine hydrochloride intermediate SM1 and related impurities 3-chloropropiophenone, propiophenone, 1-phenylpropanol, naphthol, SM1a, SM1b, SM1c, SM1d, SM1e and UI-1
[0075] (1) Preparation of positioning solutions for SM1 and impurities 3-chloropropiophenone, propiophenone, 1-phenylpropanol, naphthol, SM1a, SM1b, SM1c, SM1d, SM1e and UI-1
[0076] Preparation of 3-chloropropiophenone positioning solution: Weigh 10.55 mg of 3-chloropropiophenone, put it in a 50ml volumetric flask, add diluent to dissolve and dilute to the mark, shake well, and use it as impurity 3-chloropropiophenone stock solution; Take 1.0ml of the above-mentioned 3-chloropropiophenone stock solution, put it in a 100ml volumetric flask, add diluent to dilute to the mark, and shake well to obtain the 3-chloropropiophenone positioning solution with a concentration of 2.11 μg / ml.
[0077]Propiophenone positioning solution: Weigh 12.40mg of propiophenone, put it in a 25ml ...
Embodiment 2
[0089] Example 2 Separation of dapoxetine hydrochloride intermediate SM1, 3-chloropropiophenone, propiophenone, 1-phenylpropanol, naphthol, SM1a, SM1b, SM1c, SM1d, SM1e and UI-1
[0090] (1) Preparation of mixed solutions of SM1, 3-chloropropiophenone, propiophenone, 1-phenylpropanol, naphthol, SM1a, SM1b, SM1c, SM1d, SM1e and UI-1
[0091] Accurately pipette the SM prepared in Example 1 1a , SM 1b , SM 1c , SM 1d 1.0ml of each stock solution, 6.0ml each of SM1, 3-chloropropiophenone, propiophenone, 1-phenylpropanol, naphthol, SM1e and UI-1 stock solution, put them in the same 100ml volumetric flask, add diluent to dilute to scale, shake well, that is a mixed solution.
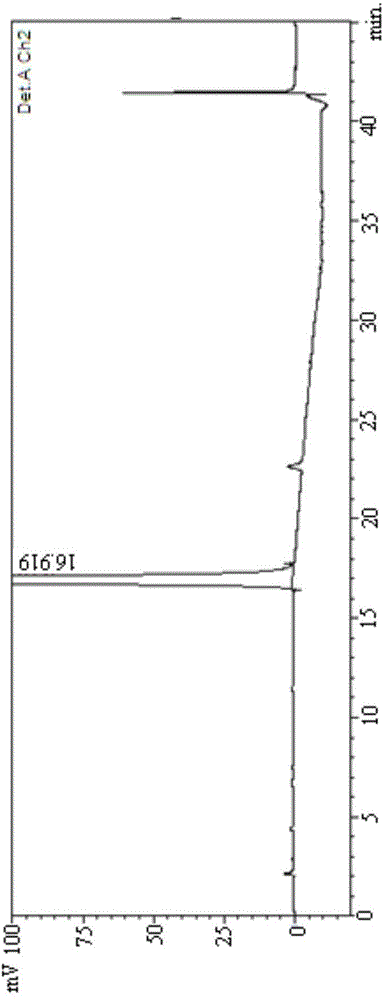
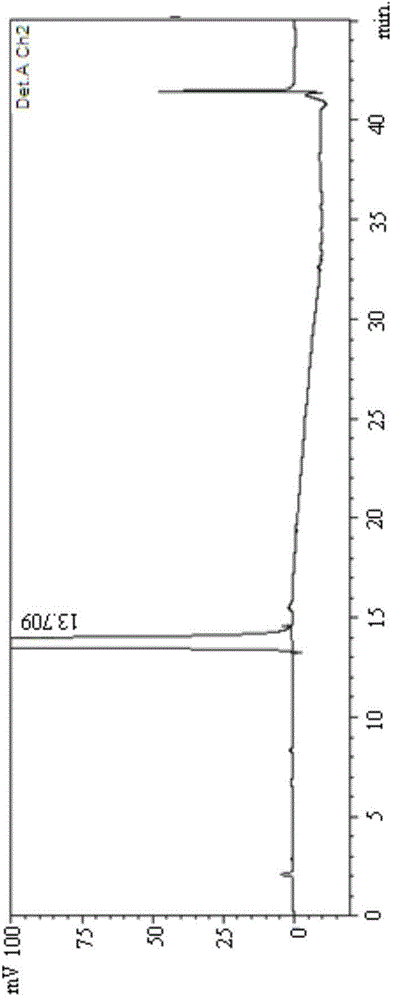
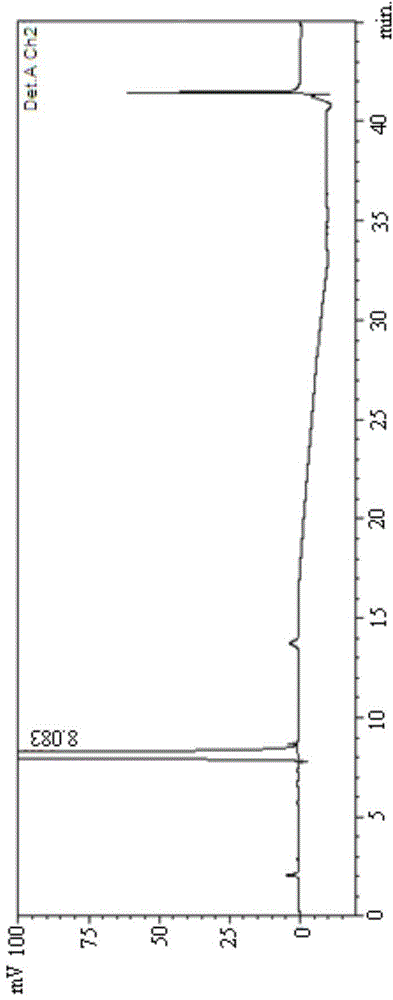
[0092] (2) With 45% acetonitrile aqueous solution (acetonitrile: water=45:55) as mobile phase, get the mixed solution that makes and analyze according to above-mentioned chromatographic conditions, record chromatogram as follows Figure 12 Shown, it can be seen that the method SM1 of the present invention...
Embodiment 3
[0093] Separation and detection of SM1, 3-chloropropiophenone, propiophenone, 1-phenylpropanol, naphthol, SM1a, SM1b, SM1c, SM1d, SM1e and UI-1 in the test sample
[0094] (1) Preparation of the test product solution: Take about 25 mg of the test product and dissolve it with a diluent to make a 0.5 mg / mL test solution.
[0095] (2) Self-contrast solution: Precisely pipette 0.5ml of the test solution prepared in step (1), put it in a 100ml volumetric flask, add diluent to dilute 200 times, and prepare a 0.0025mg / mL self-contrast solution.
[0096] (3) The mobile phase, acetonitrile aqueous solution, was subjected to gradient elution according to the elution conditions shown in Table 1.
[0097] Table 1 Gradient elution table (volume ratio of acetonitrile and water)
[0098] time (min)
water
0
45
55
13
45
55
25
70
30
30
85
15
38
85
15
38.01
45
55
45
45
55
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com