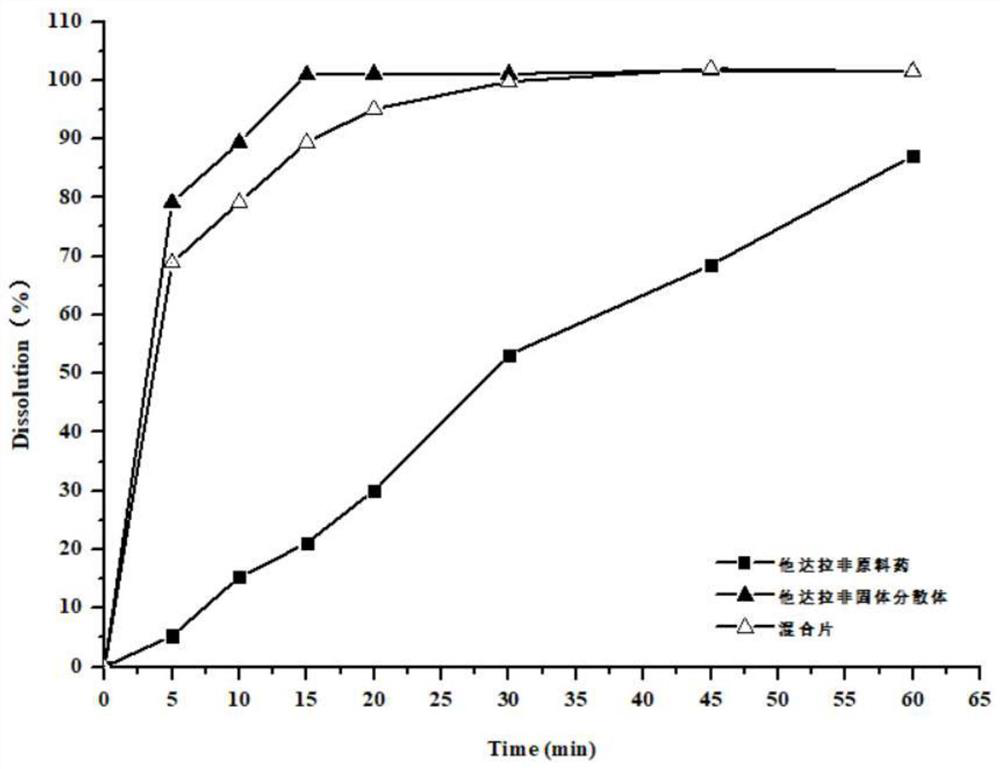
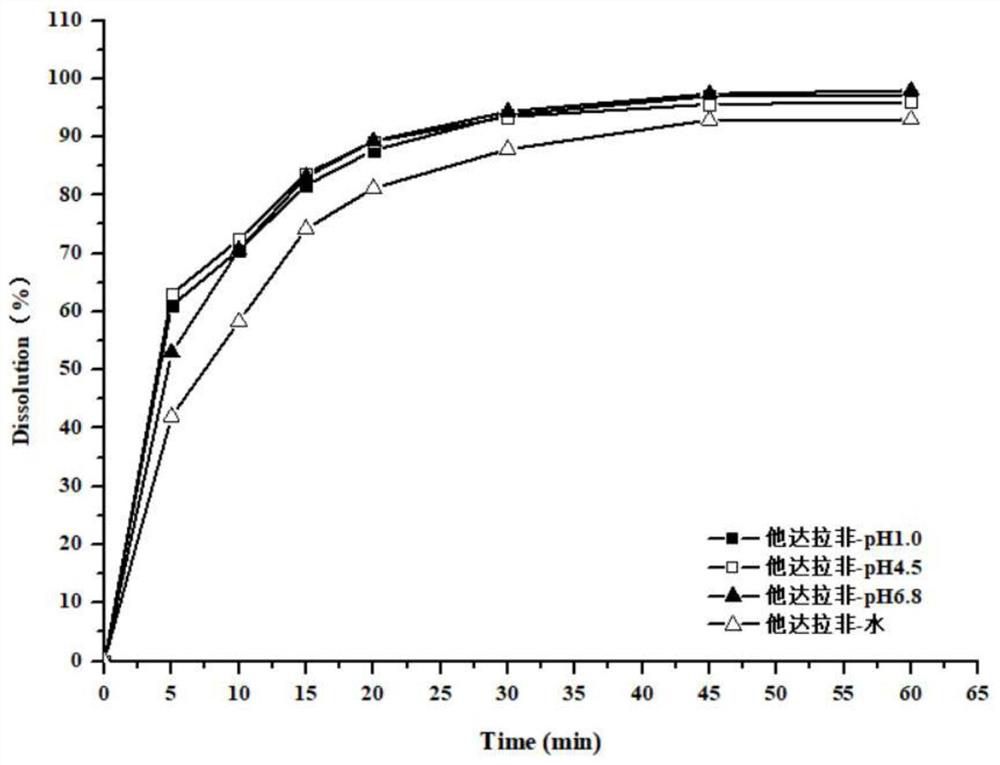
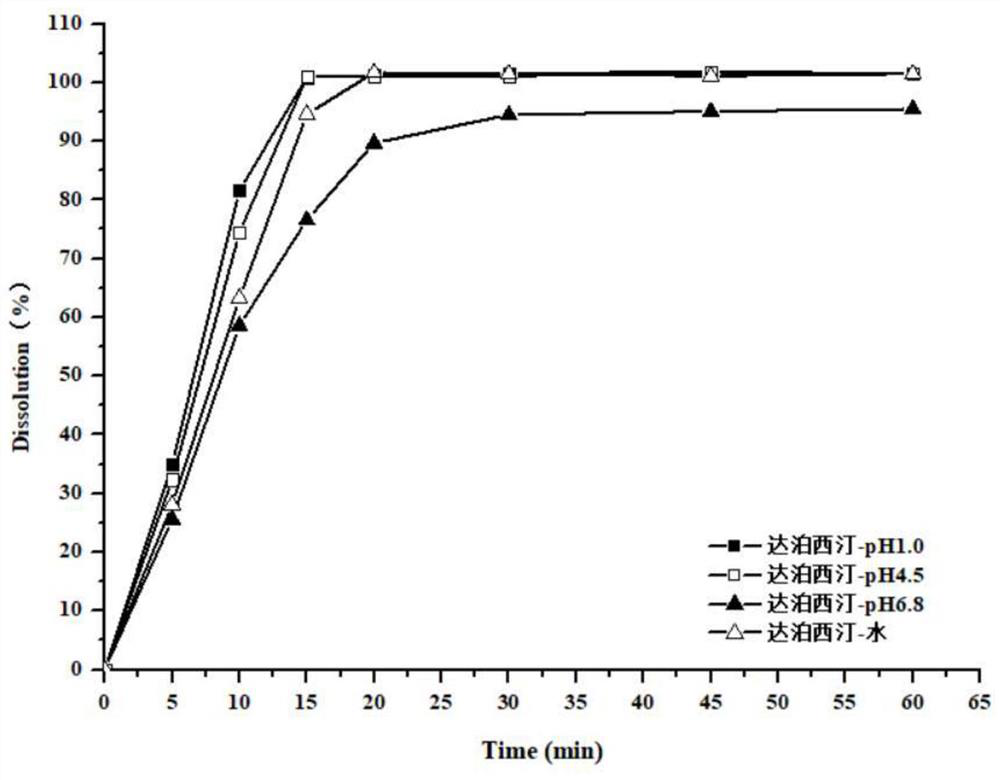
Preparation method of tadalafil and dapoxetine hydrochloride mixed tablet
A technology of dapoxetine hydrochloride and tadalafil, which is applied in the field of preparation of tadalafil and dapoxetine hydrochloride mixed tablets, can solve problems such as no research on mixed tablets, and achieve good application prospects and experimental results. Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The prescribing information is as follows:
[0060]
[0061] (1) Tadalafil, PVP K 90 Sorbitol 188 was dissolved in 80mL dimethyl sulfoxide in sequence according to the above prescription ratio (tadalafil: PVP: sorbitol = 5:13:2), and rotatively evaporated in a water bath at 60°C until the liquid became viscous , evaporate the solvent completely at 80°C, place it in a refrigerator at -20°C for 4 hours, then dry it in a vacuum oven at 50°C for 24 hours, take it out, crush it, pass through a 60-mesh sieve, and store it in a desiccator for later use.
[0062] (2) Take tadalafil solid dispersion (tadalafil content 40% w / w), dapoxetine hydrochloride, microcrystalline cellulose, lactose, croscarmellose after calculating according to the prescription quantity Mix with sodium for 15 minutes, then add micronized silica gel, and mix for 3 minutes to obtain the total mixed powder.
[0063] (3) Press the tablet with a 6mm round punch, and the hardness is controlled at 7-11kgf. ...
Embodiment 2
[0066] The prescribing information is as follows:
[0067]
[0068] (1) Tadalafil, PVP K 90 Sorbitol 188 was dissolved in 80mL dimethyl sulfoxide in sequence according to the above prescription ratio (tadalafil: PVP: sorbitol = 5:13:2), and rotatively evaporated in a water bath at 60°C until the liquid became viscous , evaporate the solvent completely at 80°C, place it in a refrigerator at -20°C for 4 hours, then dry it in a vacuum oven at 50°C for 24 hours, take it out, crush it, pass through a 60-mesh sieve, and store it in a desiccator for later use.
[0069] (2) Take tadalafil solid dispersion (tadalafil content 40% w / w), dapoxetine hydrochloride, microcrystalline cellulose, lactose, croscarmellose after calculating according to the prescription quantity Mix with sodium for 15 minutes, then add micronized silica gel, and mix for 3 minutes to obtain the total mixed powder.
[0070] (3) Press the tablet with a 5mm round punch, and the hardness is controlled at 5-9kgf. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com