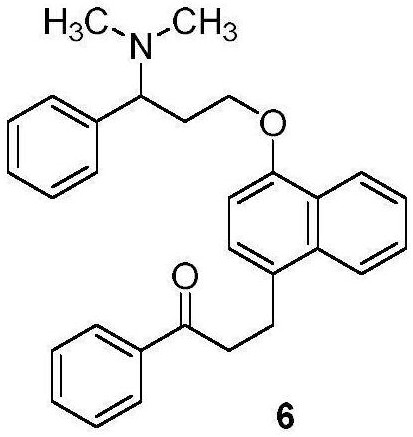
Preparation method of dapoxetine impurity reference substance
A technology of impurity reference substance, dapoxetine, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of compound 7 using p-methoxychlorbenzyl
[0057]
[0058]Compound 3 (200g, 0.718mol, 1eq.) was dissolved in dichloromethane (2000ml), triethylamine (181.6g, 248.8ml, 1.795mol, 2.5eq.) was added, and the reaction solution was cooled to below 10°C, Add p-methoxychlorbenzyl (134.9g, 0.862mol, 1.2eq) dropwise, after the reaction is over, add 10% potassium bisulfate solution (1000ml), stir and separate layers, collect the lower organic phase, add n-heptane (1000ml) , cooled to 5±5°C for crystallization, filtered, and vacuum-dried at 40±5°C to obtain 260.4 g of an off-white solid with a yield of 91%, which is compound 7.
[0059] ESI-MS(+):399.2[M+H] + .1H-NMR (400MHz, CDCl3): 8.31(d, J=5.5Hz, 1H), 8.08(d, J=5.5Hz, 1H), 7.62~7.59(m, 3H), 7.40~7.27(m, 6H ),6.98(d,J=5.5Hz,2H),6.91(d,J=5.5Hz,2H),6.42(d,J=5.0Hz,1H),4.71(s,2H),4.43(t,J =7.0Hz, 1H), 3.92(t, J=7.5Hz, 2H), 3.70(s, 3H), 2.17(t, J=7.5Hz, 2H).
Embodiment 2
[0060] Embodiment 2: use p-methoxybromide to prepare compound 7
[0061]
[0062] Compound 3 (200g, 0.718mol, 1eq.) was dissolved in dichloromethane (2000ml), triethylamine (181.6g, 248.8ml, 1.795mol, 2.5eq.) was added, and the reaction solution was cooled to below 10°C, Add p-methoxybromide (173.3g, 0.862mol, 1.2eq) dropwise, after the reaction, add 10% potassium bisulfate solution (1000ml), stir and separate layers, collect the lower organic phase, add n-heptane (1000ml) , cooled to 5±5°C for crystallization, filtered, and vacuum-dried at 40±5°C to obtain 262.7 g of an off-white solid with a yield of 91.8%, which is compound 7.
Embodiment 3
[0063] Embodiment 3: preparation compound 8
[0064]
[0065] Compound 7 (50g, 0.125mol) was dissolved in nitrobenzene (300ml), aluminum chloride (33.46g, 0.25mol, 2eq.) was added, and compound 1 (42.31g, 0.251mol, 2eq.) was dissolved in Nitrobenzene (200ml), the reaction system was heated to 100-110°C, and the solution of compound 1 was added dropwise, and the dropwise addition took 3 hours, and the resulting reaction mixture was stirred and reacted at 100-110°C for 2 hours. Monitor the reaction (TLC monitoring condition is GF254 silica gel plate, developing agent is ethyl acetate:petroleum ether (v / v)=1:20, observe with 254nm ultraviolet light) until compound 7 basically disappears, stop heating, and cool to below 20°C .
[0066] The reaction solution was added dropwise to 10% dilute hydrochloric acid solution (1000ml), the lower organic phase was separated, washed with saturated sodium chloride solution (300ml×3), the organic phase was concentrated to dryness under redu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com