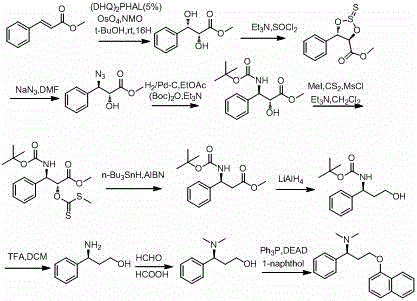
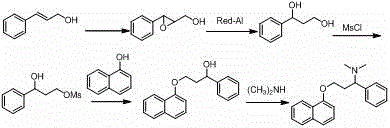
Synthesis, separation-purification, and salt forming method of dapoxetine
A technology of dapoxetine and salt formation, which is applied in the field of gradient separation, purification and salt formation, and the synthesis of dapoxetine, which can solve problems such as difficult control of sources or by-products, non-synthetic methods, long synthetic routes, etc., to achieve Promote safety and operability, improve feasibility and safety, and effect of short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
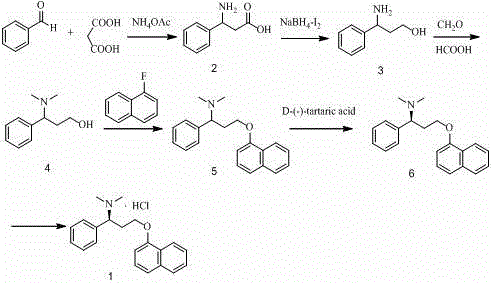
[0056] Embodiment 1: a kind of preparation method of dapoxetine comprises the following steps:
[0057] Step (1) React benzaldehyde, malonic acid and ammonium acetate in an anhydrous ethanol solvent at 75-85°C (preferably 78°C). After the reaction is complete, let it stand and cool, and filter it to obtain a white solid 3-amino-3- Phenylpropionic acid (Formula 2);
[0058] Step (2) 3-Amino-3-phenylpropionic acid, NaBH 4 Dissolve in tetrahydrofuran solvent, under ice bath condition, temperature control 0°C-10°C, slowly drop into iodine solution, after the reaction is complete, quench, evaporate the solvent under reduced pressure, treat with alkaline substances, and obtain the reduced product after extraction 3-Amino-3-phenylpropanol (Formula 3);
[0059] Step (3) Dissolve the reduction product 3-amino-3-phenylpropanol in formic acid solvent, then add formaldehyde solution, reflux at 85°C-95°C, treat with alkaline substances after the reaction is complete, and obtain the key i...
Embodiment 2
[0064] Embodiment 2: a kind of preparation method of dapoxetine, the steps are as follows:
[0065] At 75-85°C (preferably 78°C), with absolute ethanol as the solvent, add benzaldehyde, malonic acid, ammonium acetate in sequence (benzaldehyde:malonic acid:ammonium acetate is 1:1.0-1.5:1.0-1.5 moles ratio), after the reaction is complete, cool to room temperature, and filter with suction to obtain 3-amino-3-phenylpropionic acid as a white solid.
[0066] Under ice-bath conditions, sodium borohydride and 3-amino-3-phenylpropionic acid were dissolved in THF solvent, and iodine solution dissolved in THF (3-amino-3-phenylpropionic acid: boron Sodium hydride: iodine is 1:1.0-1.5:1.0-1.5 molar ratio), keep the temperature of the reaction solution not higher than 10°C, after the gas is completely released, reflux at 62°C-68°C (preferably 65°C), after the reaction is complete , quenched with anhydrous methanol, evaporated to remove the solvent, added 40% sodium hydroxide or potassium ...
Embodiment 3
[0074] Embodiment 3: a kind of preparation method of dapoxetine, the steps are:
[0075] In a 250 ml three-necked flask, use 140 ml of absolute ethanol as a solvent, add 22.13 g of malonic acid (0.21 mol), 16.35 g of ammonium acetate (0.21 mol), and 15.0 g of benzaldehyde (0.14 mol), and control the temperature at 78°C , Condensed and refluxed, reacted for 6h, monitored by TLC, showed that there was no raw material, after the reaction was completed, cooled to room temperature, filtered, washed with absolute ethanol to obtain 16.0 g of white solid 3-amino-3-phenylpropionic acid, mp 213.0 -213.6℃, MS m / z:166[M+H] + .
[0076]Under ice bath conditions, add 1.38 g (0.036 mol) of sodium borohydride, 4 g (0.024 mol) of the compound 3-amino-3-phenylpropionic acid and THF (65 ml) into a 250 ml three-necked flask, and stir After 10 min, slowly add iodine (6.16 g, 0.024 mol) dissolved in THF (30 ml) dropwise from the constant pressure dropping funnel, keep the temperature of the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com