High-purity dapoxetine preparation method suitable for industrialization
A technology for dapoxetine hydrochloride and compounds, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxy compounds, etc., can solve the problems of high cost, harsh reaction conditions, complicated routes, etc., and achieves low cost and conversion. The effect of high rate and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
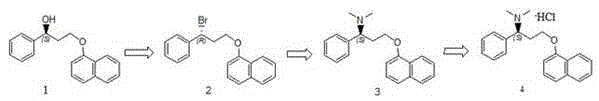
[0047] Compound 1 (1.0g) was dissolved in 10ml of dichloromethane, under the protection of nitrogen, the temperature was controlled at -5~5°C, triphenylphosphine (1.7g) was added, and N-bromosuccinimide (1.1g ), reacted for 4h, and monitored the reaction by HPLC until compound 3 was completely reacted to generate compound 2.
Embodiment 2
[0049] 10eq of dimethylamine (1.7g) was added to the reaction system of Example 1, and the reaction temperature was 5°C. The reaction was monitored for completion by HPLC. They were washed with alkaline water, water and saturated brine respectively, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to obtain compound 3 (0.84 g), with a yield of 87.9%.
Embodiment 3
[0051] Compound 3 (0.76g) was dissolved in ethyl acetate, and hydrogen chloride gas was passed through until no precipitation occurred, and filtered, and the resulting solid was recrystallized from isopropanol to obtain compound 4 (0.78g), namely dapoxetine hydrochloride, with a yield of 91.8 %, purity 99.9%, chiral purity 99.9%.
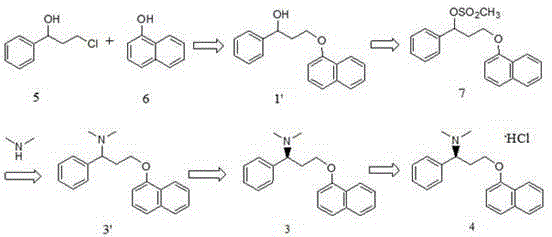
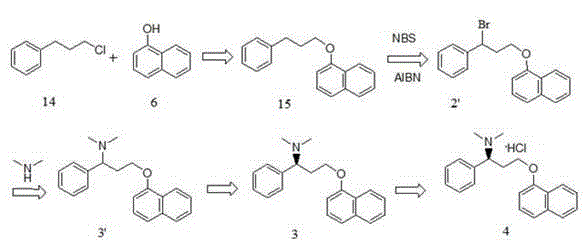
[0052] Route B:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com