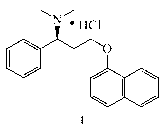
Crystal form B of dapoxetine hydrochloride, and preparation method thereof
A technology of dapoxetine hydrochloride and crystal form, which is applied in the field of organic chemistry, can solve the problem of no literature report on the crystal form and the preparation method of the crystal form, and achieves the effects of good preparation adaptability and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of Dapoxetine Hydrochloride Form B.
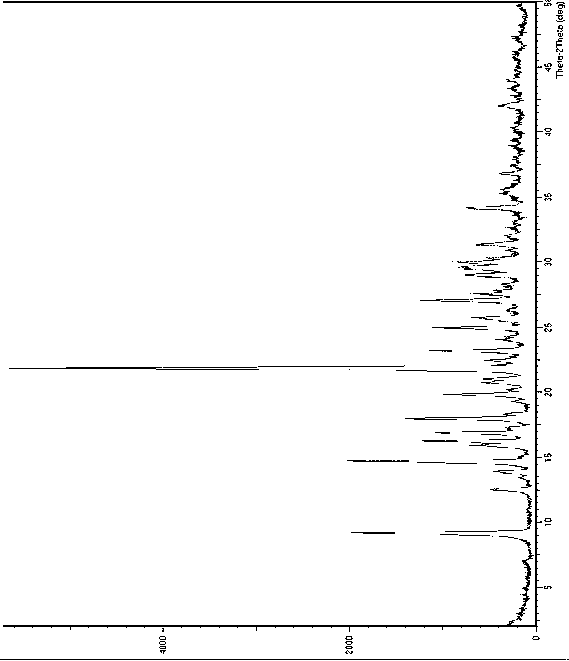
[0045] Put 5g of dapoxetine hydrochloride in a reaction vessel, add 5ml of water under stirring, after the addition is complete, cool to 5-10°C and stir for about 30 minutes, filter to obtain dapoxetine hydrochloride crystal form B, and its X-ray powder diffraction See atlas Figure 1 , the main test data values are listed in the following table (list the test data with relative strength greater than or equal to 5%).
[0046] 2θ angle (°) measured value d(?) Measured value Relative Strength(%) 9.17060 9.63559 35 12.5068 7.07178 8 13.8561 6.38602 6 14.6749 6.03150 35 15.8904 5.57276 9 16.2194 5.46045 19 16.8657 5.25264 17 17.9705 4.93212 24 19.8103 4.47803 14 20.7525 4.27680 8 21.0175 4.22347 7 21.8331 4.06750 100 22.4313 3.96036 6 23.1739 3.83511 18 24.9375 3.56774 17 25.6766 3.46669 9 27.0515 3.29354 20 ...
Embodiment 2
[0049] Preparation of Dapoxetine Hydrochloride Form B
[0050] Put 3g of dapoxetine hydrochloride in a reaction vessel, add 6ml of 20% isopropanol aqueous solution under stirring, after the addition is completed, stir at room temperature for about 50min, filter to obtain dapoxetine hydrochloride crystal form B, X-ray powder diffraction pattern within the margin of error with figure 1 unanimous.
[0051]
Embodiment 3
[0053] Preparation of Dapoxetine Hydrochloride Form B
[0054] Put 6g of dapoxetine hydrochloride in a reaction vessel, add 24ml of 40% tetrahydrofuran aqueous solution under stirring, after the addition is complete, let it stand and cool to 0~5°C for about 1.5h, filter to obtain dapoxetine hydrochloride crystal form B, X - X-ray powder diffraction pattern within error with figure 1 unanimous.
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com