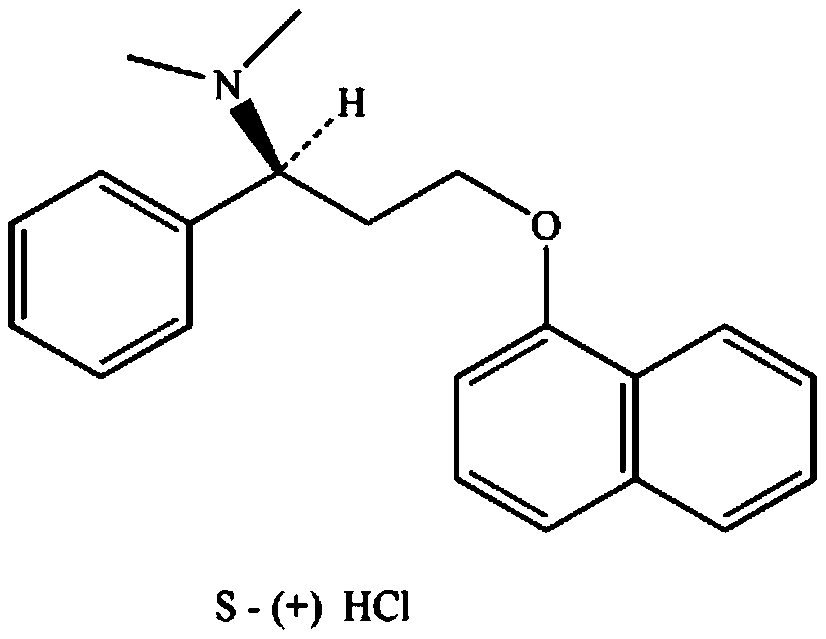
Dapoxetine hydrochloride tablet and preparation method thereof
A technology for dapoxetine hydrochloride and cetine tablets, which can be applied in the fields of pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as complex preparation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
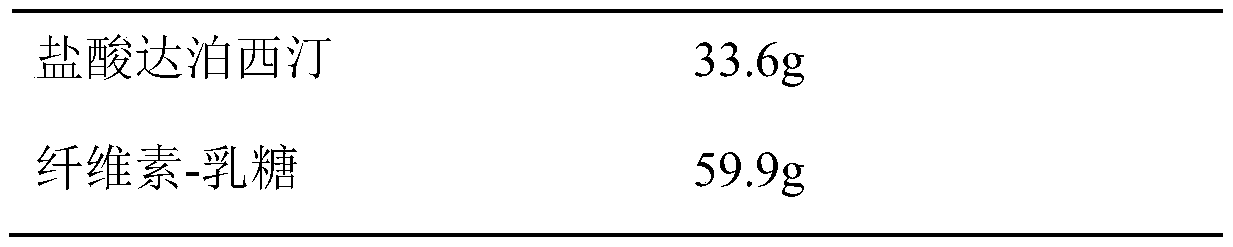
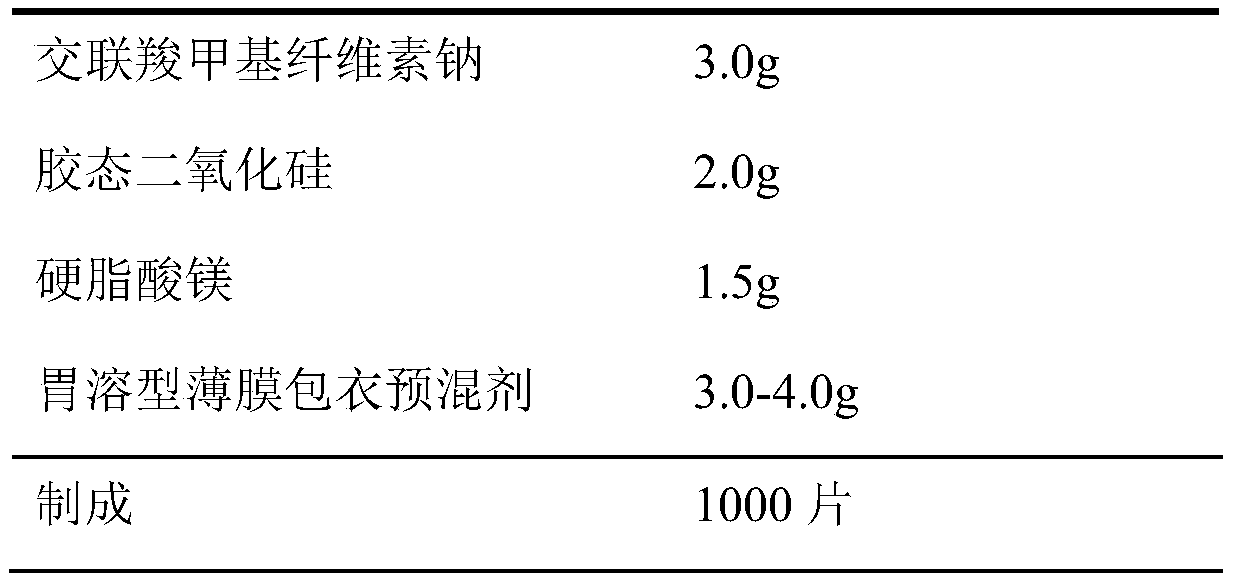
[0017] prescription
[0018]
[0019]
[0020] Preparation Process:
[0021] ①Pretreatment: Put dapoxetine hydrochloride in a high-efficiency pulverizer to pulverize through a 0.6mm sieve, and colloidal silica through a 24-mesh sieve for treatment. ② Mixing: Mix dapoxetine hydrochloride, cellulose-lactose, croscarmellose sodium, and colloidal silicon dioxide for 5 minutes first, then add magnesium stearate, and mix for another 1 minute. ③ Tabletting: Compress the mixed powder into tablets. ④ Coating: adding the coating powder into purified water to prepare a coating liquid with a solid content of 12%, and then coating, the weight of the coating increases by 3-4%. ⑤Aluminum / plastic / aluminum packaging: the coated tablets are packaged in aluminum / plastic / aluminum.
Embodiment 2
[0023] prescription
[0024]
[0025] Preparation Process:
[0026] ①Pretreatment: Put dapoxetine hydrochloride in a high-efficiency pulverizer to pulverize through a 0.6mm sieve, and colloidal silica through a 24-mesh sieve for treatment. ② Mixing: Mix dapoxetine hydrochloride, cellulose-lactose, sodium carboxymethyl starch, and colloidal silicon dioxide for 5 minutes first, then add magnesium stearate, and mix for another 1 minute. ③ Tabletting: Compress the mixed powder into tablets. ④ Coating: adding the coating powder into purified water to prepare a coating liquid with a solid content of 12%, and then coating, the weight of the coating increases by 3-4%. ⑤Aluminum / plastic / aluminum packaging: the coated tablets are packaged in aluminum / plastic / aluminum.
Embodiment 3
[0028] prescription
[0029]
[0030] Preparation Process:
[0031] ①Pretreatment: Put dapoxetine hydrochloride in a high-efficiency pulverizer to pulverize through a 0.6mm sieve, and colloidal silica through a 24-mesh sieve for treatment. ② Mixing: Mix dapoxetine hydrochloride, cellulose-lactose, croscarmellose sodium, and colloidal silicon dioxide for 5 minutes first, then add magnesium stearate, and mix for another 1 minute. ③ Tabletting: Compress the mixed powder into tablets. ④ Coating: adding the coating powder into purified water to prepare a coating liquid with a solid content of 12%, and then coating, the weight of the coating increases by 3-4%. ⑤Aluminum / plastic / aluminum packaging: the coated tablets are packaged in aluminum / plastic / aluminum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com