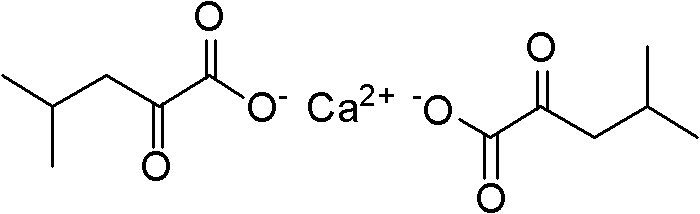Method for synthesizing alpha-ketoleucine calcium
A technology of ketoleucine calcium and a synthesis method, which is applied in the field of pharmaceutical raw materials, can solve the problems of difficult hydrolysis of 5-benzylidene hydantoin, short reaction steps, and long reaction time, and achieve easy control of the reaction, easy hydrolysis, The effect of small investment in equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
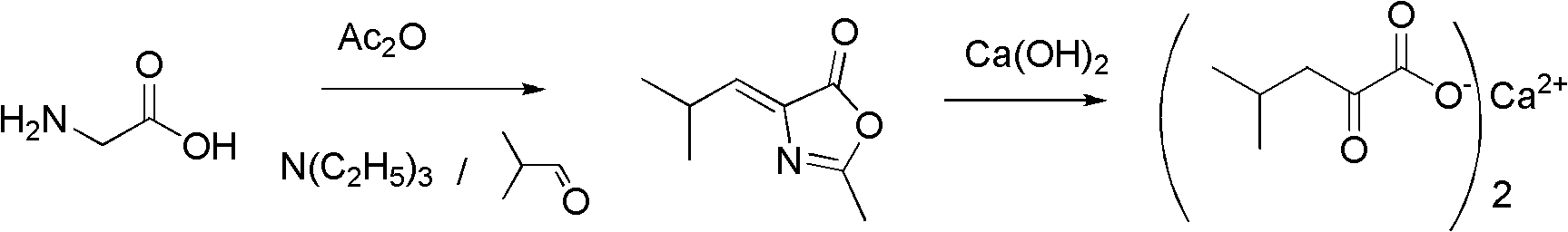
[0044] Example 1: Preparation of 4-isobutylene-2-methyldihydrooxazolone
[0045] Put 37.5g (0.5mol) of pharmaceutical grade glycine and 50.5g (0.5mol) of triethylamine into a 500ml three-necked flask equipped with mechanical stirring, thermometer, and reflux condenser, stir evenly, and add dropwise at 50°C while stirring and cooling 127.5g (1.25mol) of acetic anhydride, the dropwise addition of acetic anhydride is completed, and the reaction is stirred at 40-50°C for 1 hour, then 36g (0.5mol) of isobutyraldehyde is added dropwise in 15 minutes, and the temperature is raised to 60-65°C for reaction After 3 hours, heat up to 90°C in 3 hours, and continue to react at 90-100°C for 3 hours until the peak of isobutyraldehyde disappears. After the reaction is completed, the mixture of isobutyraldehyde, acetic anhydride, acetic acid, and triethylamine is distilled off under reduced pressure. After distillation, it can be used in the next batch of reactions. The residue is cooled to a ...
Embodiment 2-6
[0046] Example 2-6: Preparation of 4-isobutylidene-2-methyldihydrooxazolone under the same conditions as Example 1 except that different catalysts are used.
[0047]
Embodiment 7
[0048] Example 7: Preparation of 4-isobutylene-2-methyldihydrooxazolone
[0049] In a 500ml three-necked flask equipped with a mechanical stirrer, a thermometer, and a reflux condenser, put 37.5g (0.5mol) of pharmaceutical grade glycine and 33.3g (0.25mol) of N,N'-dimethylpiperazine, stir well, and cool Add 127.5g (1.25mol) of acetic anhydride dropwise under control at 50°C. After the dropwise addition of acetic anhydride is complete, stir and react at 40-50°C for 1 hour, then add 36g (0.5mol) of isobutyraldehyde dropwise in 15 minutes. Raise the temperature to 60-65°C for 3 hours, then raise the temperature to 90°C for 3 hours, continue to react at 90-100°C for 3 hours until the peak of isobutyraldehyde disappears, and the reaction is completed. The mixture was dissolved by adding 500 mL of toluene to the residue, washed twice by adding 100 mL of water each time, the toluene solution was evaporated to dryness under reduced pressure, and cooled to obtain a solid of 4-isobutyli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com