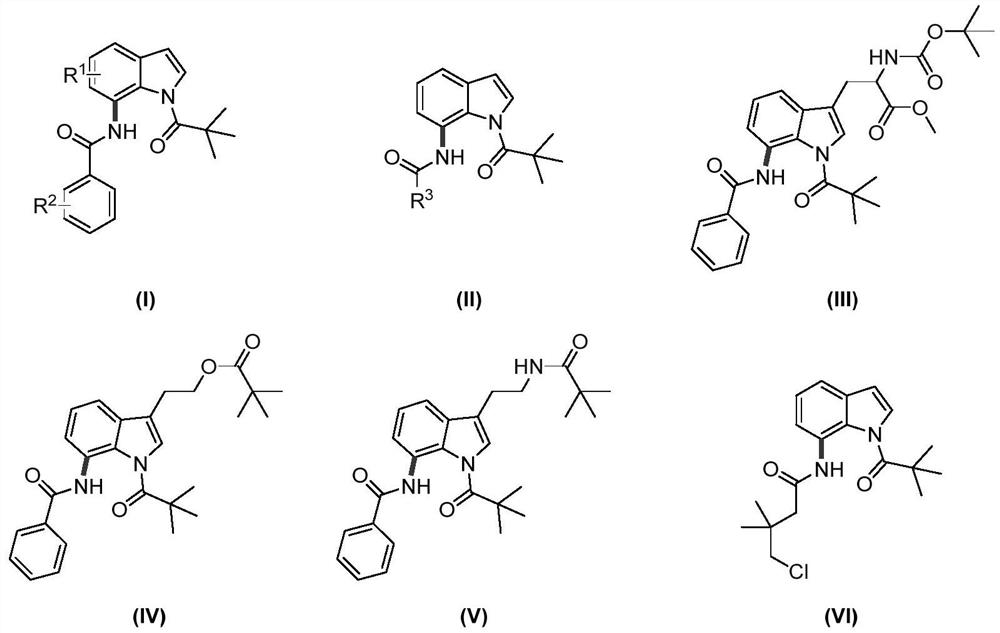
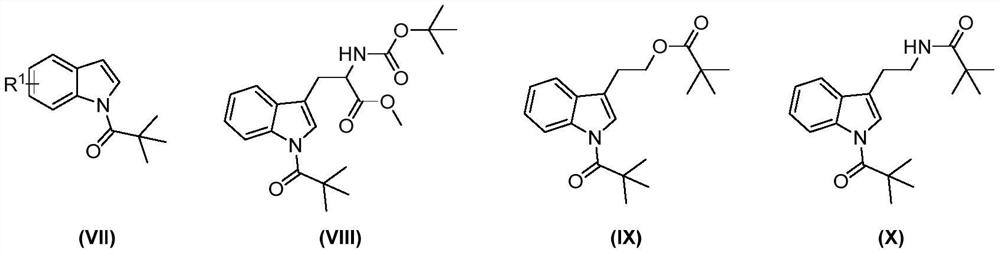
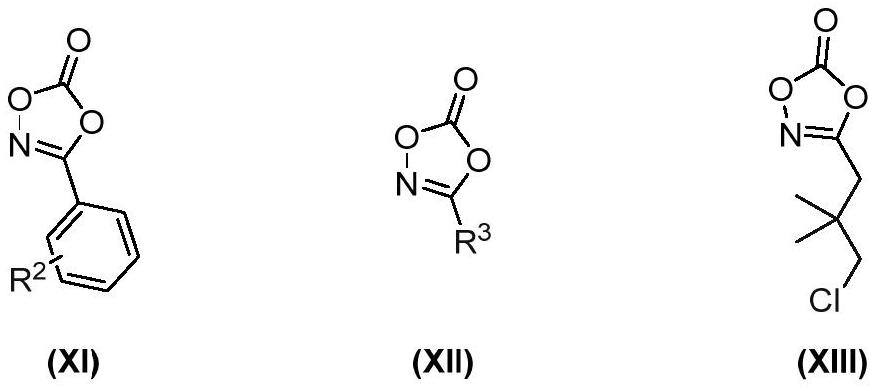
Preparation method of 7-amide indole compound
A compound and indole technology, which is applied in the field of ruthenium-catalyzed preparation of 7-amide indole compounds, and achieves the effects of simple preparation method, low reaction cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] At room temperature, N-pivaloyl indole (0.2mmol), 3-phenyloxazolone (0.6mmol), [Ru(p-cymene)Cl 2 ] 2 (5mol%), AgSbF 6 (20mol%), pivalic acid (3 equiv.) and HFIP (2.0 mL). Stir at room temperature. TLC tracking detection reaction. After 24 hours, the reaction was stopped. Water and dichloromethane were added to the reaction system, the organic layer was separated, and the aqueous layer was washed three to five times with dichloromethane. All organic layers were combined, dried with anhydrous sodium sulfate, concentrated, separated by column chromatography (5% ethyl acetate petroleum ether solution), obtained 43.6 mg of product, and the yield was 68%. The reaction process is shown in the following formula:
[0030]
[0031] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0032] 1 H NMR (600MHz, CDCl 3 )δ10.30(brs,1H),8.22(dd,J=5.9,3.2Hz,1H),8.11–8.09(m,2H),7.67(d,J=3.9Hz,1H),7.56–7.50(m ,3H),7.40–7.38(m,2H),6.68...
Embodiment 2
[0034]At room temperature, N-pivaloyl indole (0.2mmol), 3-phenyloxazolone (0.6mmol), [Cp*IrCl 2 ] 2 (5mol%), AgSbF 6 (20mol%), pivalic acid (3 equiv.) and HFIP (2.0 mL). Stir at room temperature. TLC tracking detection reaction. After 24 hours, the reaction was stopped. Water and dichloromethane were added to the reaction system, the organic layer was separated, and the aqueous layer was washed three to five times with dichloromethane. All organic layers were combined, dried with anhydrous sodium sulfate, concentrated, and separated by column chromatography (5% ethyl acetate petroleum ether solution) to obtain 13.5 mg of product with a yield of 21%. The reaction process is shown in the following formula:
[0035]
[0036] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0037] 1 H NMR (600MHz, CDCl 3 )δ10.30(brs,1H),8.22(dd,J=5.9,3.2Hz,1H),8.11–8.09(m,2H),7.67(d,J=3.9Hz,1H),7.56–7.50(m ,3H),7.40–7.38(m,2H),6.68(d,J=3....
Embodiment 3
[0039] At room temperature, N-pivaloyl indole (0.2mmol), 3-phenyloxazolone (0.6mmol), [Ru(p-cymene)Cl 2 ] 2 (5mol%), AgSbF 6 (20mol%), benzoic acid (3 equiv.) and HFIP (2.0 mL). Stir at room temperature. TLC tracking detection reaction. After 24 hours, the reaction was stopped. Water and dichloromethane were added to the reaction system, the organic layer was separated, and the aqueous layer was washed three to five times with dichloromethane. All organic layers were combined, dried with anhydrous sodium sulfate, concentrated, separated by column chromatography (5% ethyl acetate petroleum ether solution), obtained 22.4 mg of product, and the yield was 35%, and the reaction process was shown in the following formula:
[0040]
[0041] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0042] 1 H NMR (600MHz, CDCl 3 )δ10.30(brs,1H),8.22(dd,J=5.9,3.2Hz,1H),8.11–8.09(m,2H),7.67(d,J=3.9Hz,1H),7.56–7.50(m ,3H),7.40–7.38(m,2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com