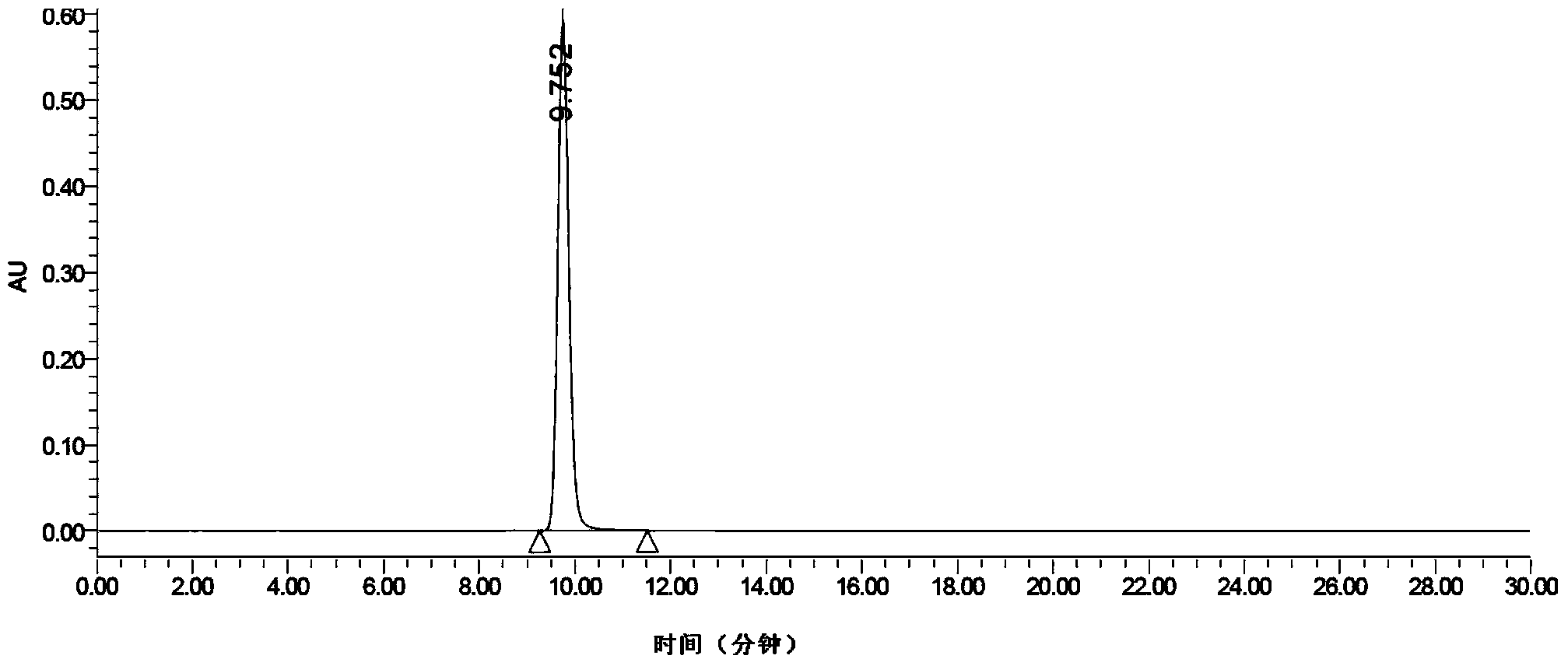
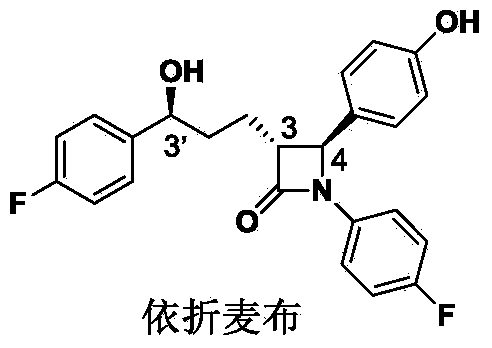
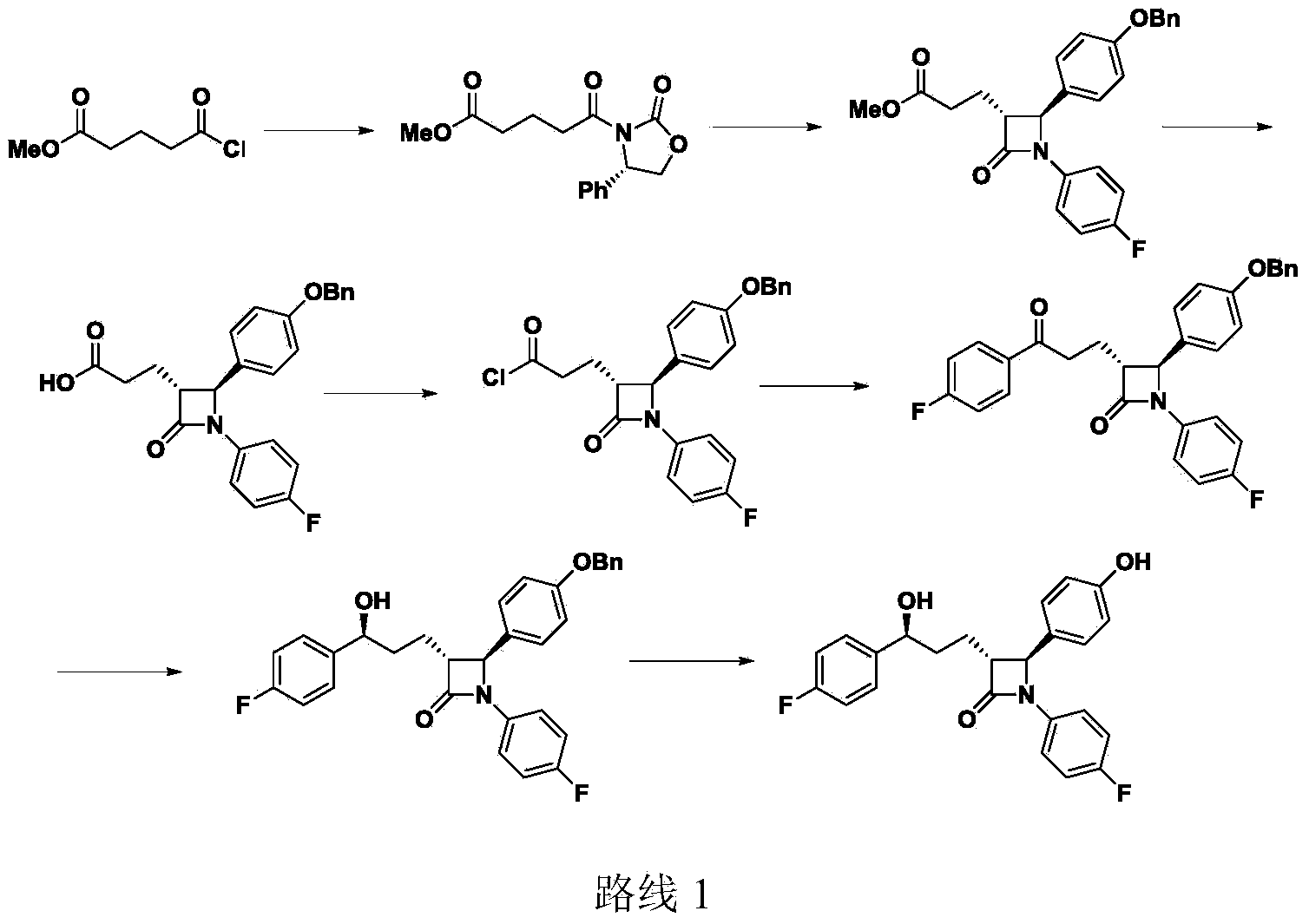
Preparation midbody for Ezetimibe and preparation method of preparation midbody
A technology for ezetimibe and intermediates, which is applied in the field of preparation of lipid-lowering drug-ezetimibe and its preparation, which can solve the problems of low total yield, limited industrial production, and easy ring opening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1. The synthesis of compound IV
[0033]
[0034] Add 5-(4-fluorophenyl)-5-oxopentanoic acid (20g, 95mmol), (S)-4-(2-chlorophenyl)-2-oxazolone (19g, 96mmol) into a 500ml three-necked flask ), N,N'-dicyclohexylcarbodiimide (19.8g, 96mmol) and dichloromethane 200ml, stirred at room temperature for 12 hours. Filter and wash the organic layer with 3% dilute hydrochloric acid (80ml). The organic layer was concentrated and the product was crystallized from isopropanol (150ml), filtered and dried. 31 g of the product was obtained with a yield of 83.7%. 1 H NMR (400MHz, CDCl 3 ):δ2.05(m,2H),2.94(m,2H),3.05(m,2H),4.26(m,1H),4.67(t,1H),5.42(m,1H),7.08(m, 2H),7.30(m,4H),7.89(m,2H).
Embodiment 2
[0035] Embodiment 2. Synthesis of Compound II
[0036]
[0037]Dry dichloromethane (20ml) and borane dimethyl sulfide (2.82ml, 28.2mmol) were added to a 250ml three-necked flask, and the mixture was cooled to -5-0°C. Add (R)-MeCBS toluene solution (1.4ml, 1.4mmol, 5%mol), and stir at 0°C for 15 minutes, slowly add compound IV (10g, 25.7mmol) in dichloromethane within 3-4 hours (30ml) solution, the reaction temperature is controlled at -5~0 degree. Stirring was continued for 1-2 hours. The reaction was quenched by the slow addition of methanol (4ml) while maintaining the temperature below 0°C. 5% hydrogen peroxide (20ml) was added followed by 4N sulfuric acid (1.5ml). The mixture was stirred for 15 minutes, the organic layer was separated and washed with 2N sulfuric acid (20ml), 5% sodium bisulfite (50ml) and 10% sodium chloride (50ml). The organic layer was concentrated to low volume until the water content was less than 0.05%. The product was used directly in the next...
Embodiment 3
[0038] Example 3. Synthesis of compound I (PG trimethylsilyl)
[0039]
[0040] A dichloromethane solution of compound II (10 g equivalent of compound 6, 25 mmol) and compound III (12.05 g) obtained from the previous step were added to a 500 ml three-necked flask, and the total volume of the reaction mixture was adjusted to 150 ml using anhydrous dichloromethane. The mixture was cooled to 1 H NMR (400MHz, CDCl3): δ-0.07(s,9H),0.28(s,9H),1.41(m,1H),1.55(m,3H),4.21(m,1H),4.29(m,1H ),4.46(m,2H),4.47(m,1H),5.43(m,1H),6.43(m,2H),6.77(m,4H),6.98(m,2H),7.06(m,2H) ,7.18(m,7H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com