Novel preparation method of netaglinide oxazolone
A technology of linzolid and its compound, which is applied in the field of drug synthesis, can solve the problems of unfavorable industrial production, serious environmental pollution, and long reaction route, and achieve the effects of high stereoselectivity, high atom utilization rate, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
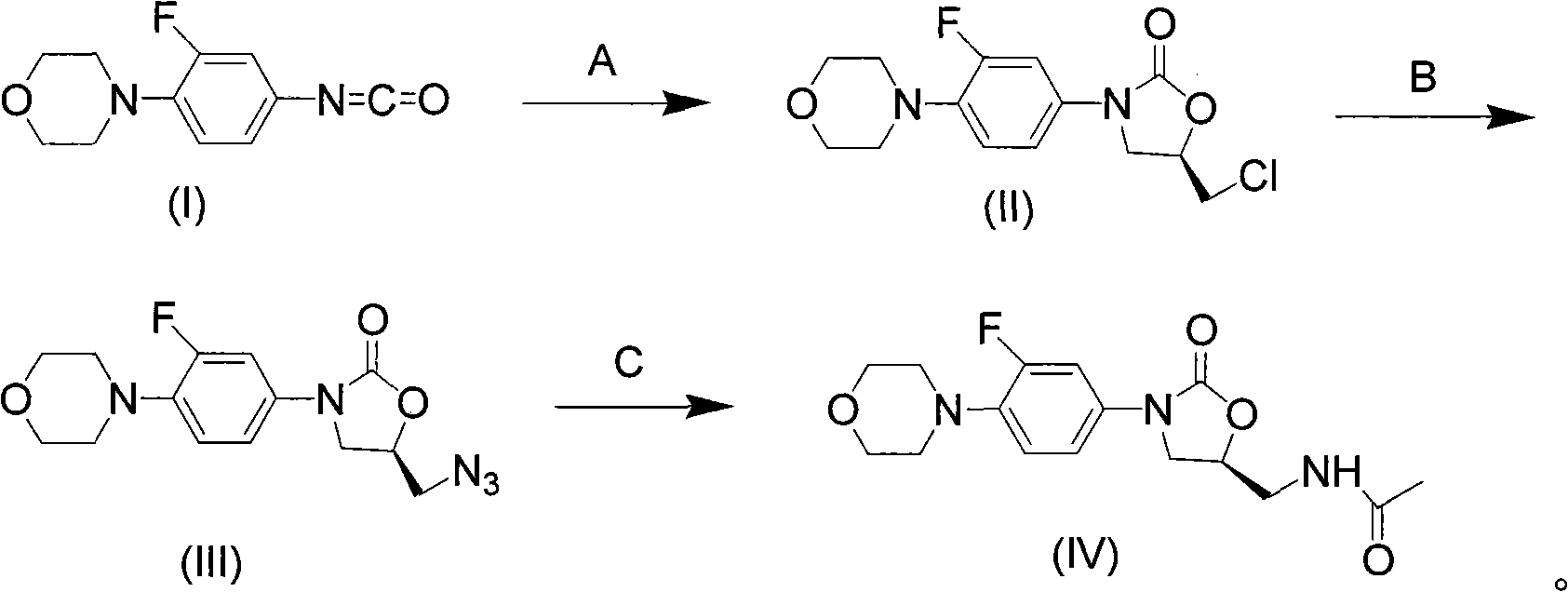
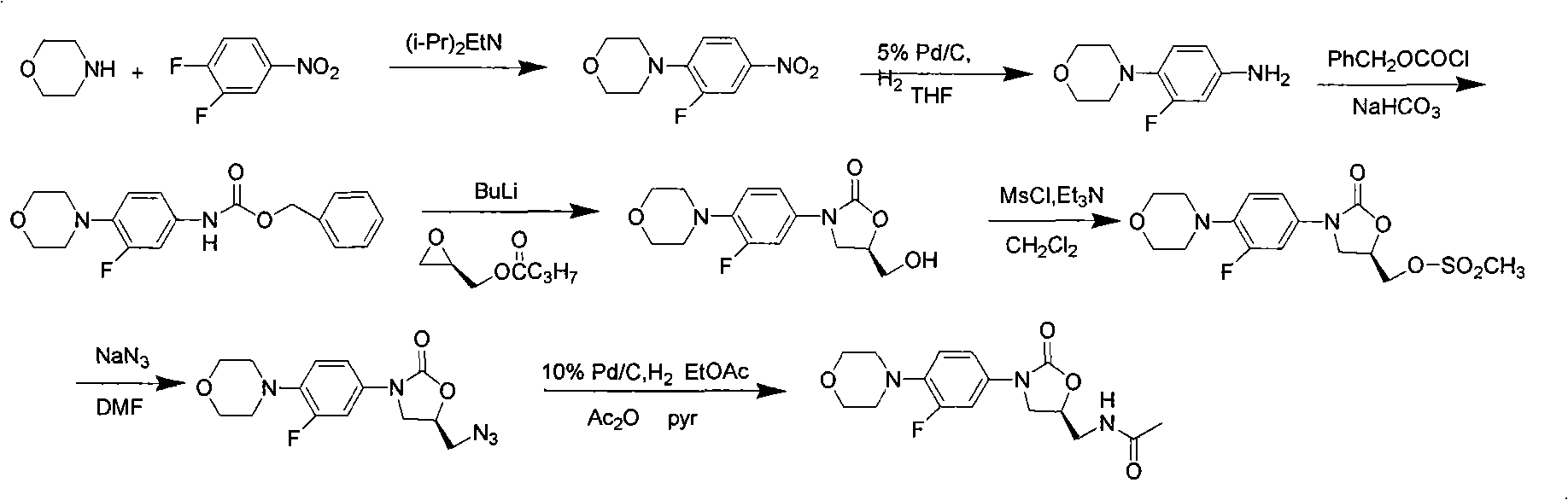
[0044] The synthesis of embodiment 13-fluoro-4-morpholine phenyl oxazolidinone
[0045] Weigh 3-fluoro-4-morpholine phenyl isocyanate (1.11g, 5mmol) and (R)-epichlorohydrin (0.51g, 5.5mmol) in a 25mL single-necked flask, add 10mL tetrahydrofuran and stir to dissolve, then add MgI 2 (2.5mmol), stirred at 50°C for 4h, added 5% sodium thiosulfate aqueous solution to quench the reaction, extracted with dichloromethane, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and the crude product was extracted with ethyl acetate-petroleum ether (V / V=1:15) recrystallized to obtain off-white solid 1.47g, yield 93%.
Embodiment 23
[0046] The synthesis of embodiment 23-fluoro-4-morpholine phenyl oxazolidinone
[0047] Weigh 3-fluoro-4-morpholine phenyl isocyanate (1.11g, 5mmol) and (R)-epichlorohydrin (0.51g, 5.5mmol) in a 25mL single-necked flask, add 10mL tetrahydrofuran and stir to dissolve, then add MgBr 2 (2.5mmol), heated and stirred at 50°C for 5h, added 5% sodium thiosulfate aqueous solution, extracted with dichloromethane, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and the crude product was washed with ethyl acetate-petroleum ether (V / V=1:15) recrystallized to obtain off-white solid 1.50 g, yield 95%.
Embodiment 33
[0048] The synthesis of embodiment 3-fluoro-4-morpholine phenyl oxazolidinone
[0049] Weigh 3-fluoro-4-morpholine phenyl isocyanate (1.11g, 5mmol) and (R)-epichlorohydrin (0.51g, 5.5mmol) in a 25mL single-necked flask, add 10mL tetrahydrofuran and stir to dissolve, then add MgCl 2 (2.5mmol), heated and stirred at 50°C for 8h, added 5% sodium thiosulfate aqueous solution, extracted with dichloromethane, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and the crude product was washed with ethyl acetate-petroleum ether (V / V=1:15) recrystallized to obtain off-white solid 0.94g, yield 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com