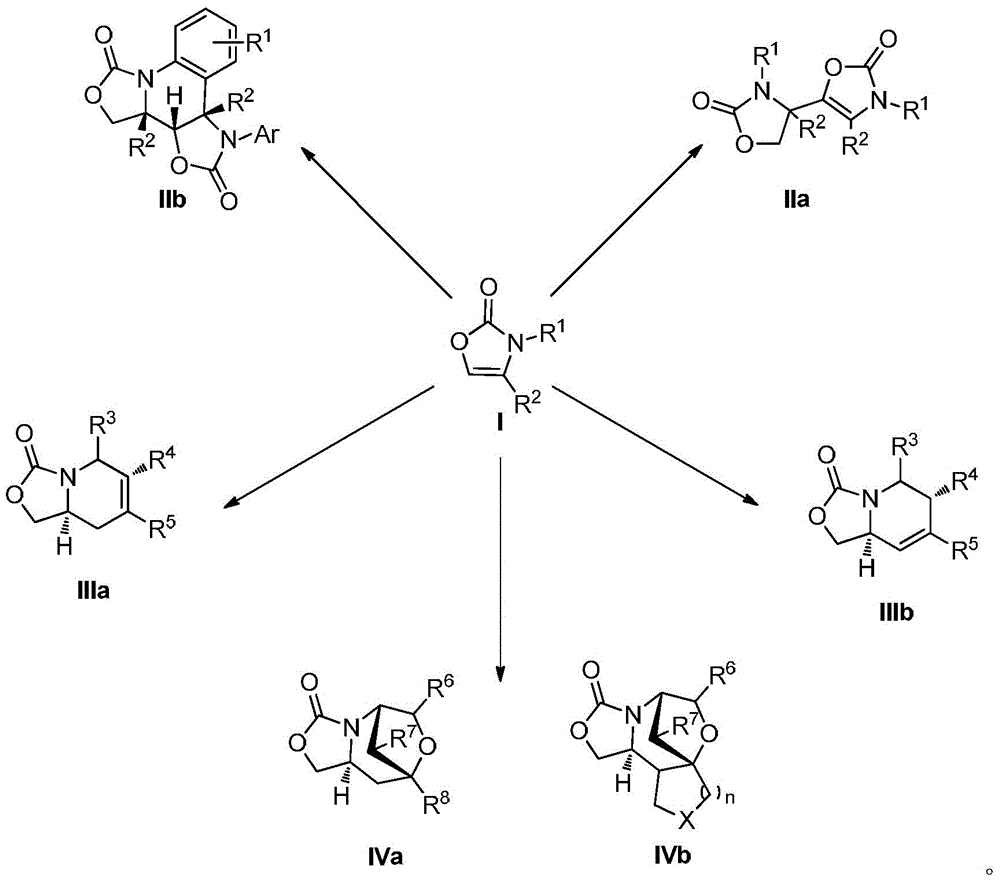
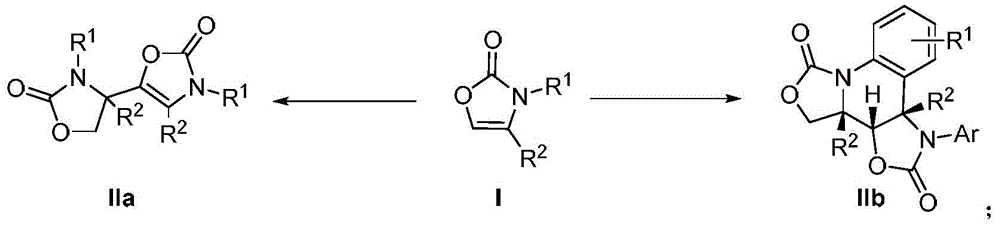
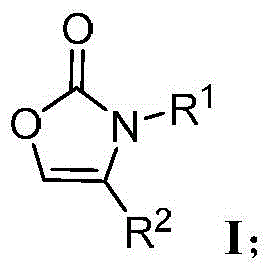
Preparation method for oxazolone heterocyclic compounds
A technology for heterocyclic compounds and oxazolones, applied in the field of oxazolone heterocyclic compounds and preparation, can solve the problems of long reaction time, low reaction selectivity and high reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: 3-allyl-5-(3-allyl-2-oxooxazolidin-4-yl)oxazol-2(3H)-one
[0078]
[0079] Boron trifluoride ether solution (41 μL, 0.5 equiv.) was added dropwise to reaction substrate I-1 (77.6 mg, 0.62 mmol) in dichloromethane solution (2 ml), and the tube was sealed. The reaction mixture was stirred at 50°C for 12 hours. After the completion of the reaction was monitored with a TLC plate, the reaction was quenched with saturated sodium bicarbonate (5 ml) and stirred for 5 minutes. Dichloromethane was extracted 3 times (5mL×3), the organic phase was mixed and washed with water (10mL) and saturated brine (10mL) respectively, and finally dried over anhydrous sodium sulfate, filtered and then rotovaped to obtain a crude product. The crude product was subjected to silica gel column chromatography to obtain pure product IIa-1 (yield 98%).
[0080] Colorless oily liquid; Rf=0.21 (petroleum ether / ethyl acetate=3 / 2); IR(KBr)ν max 3198,3163,1746,1398,756cm -1 ; 1 H NMR (400...
Embodiment 2
[0081] Example 2: 5-(2-oxo-3-propyloxazolidin-4-yl)-3-propyloxazol-2(3H)-one
[0082]
[0083] Boron trifluoride diethyl ether solution (41 μL, 0.5 equiv.) was added dropwise to reaction substrate I-2 (79 mg, 0.62 mmol) in dichloromethane solution (2 ml), and the tube was sealed. The reaction mixture was stirred at 50°C for 12 hours. After the completion of the reaction was monitored with a TLC plate, the reaction was quenched with saturated sodium bicarbonate (5 ml) and stirred for 5 minutes. Dichloromethane was extracted 3 times (5mL×3), the organic phase was mixed and washed with water (10mL) and saturated brine (10mL) respectively, and finally dried over anhydrous sodium sulfate, filtered and then rotovaped to obtain a crude product. The crude product was subjected to silica gel column chromatography to obtain pure product IIa-2 (yield 85%).
[0084] Colorless oily liquid; Rf=0.22 (petroleum ether / ethyl acetate=3 / 2); IR(KBr)ν max 3135, 2966, 1751, 1400, 1131, 754cm ...
Embodiment 3
[0085] Example 3: 3-Benzyl-5-(3-benzyl-2-oxooxazolidin-4-yl)oxazol-2(3H)-one
[0086]
[0087] Boron trifluoride diethyl ether solution (41 μL, 0.5 equiv.) was added dropwise to reaction substrate I-3 (109 mg, 0.62 mmol) in dichloromethane solution (2 ml), and the tube was sealed. The reaction mixture was stirred at 50°C for 12 hours. After the completion of the reaction was monitored with a TLC plate, the reaction was quenched with saturated sodium bicarbonate (5 ml) and stirred for 5 minutes. Dichloromethane was extracted 3 times (5mL×3), the organic phase was mixed and washed with water (10mL) and saturated brine (10mL) respectively, and finally dried over anhydrous sodium sulfate, filtered and then rotovaped to obtain a crude product. The crude product was subjected to silica gel column chromatography to obtain pure product IIa-3 (yield 87%).
[0088] White solid (melting point: 125-126°C); Rf=0.33 (petroleum ether / ethyl acetate=3 / 2); IR(KBr)ν max 3239,3093,1748,1400,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com