Preparation method of lithium ion battery electrolyte salt LiODFB (lithium oxalyldifluroborate)
A technology of lithium oxalate difluoroborate and lithium oxalate difluoroborate is applied in the field of complete process for the preparation of lithium oxalate difluoroborate (LiODFB), which can solve the problems of unfavorable industrialization, high equipment requirements, and low sample purity. Achieve the effect of avoiding waste and improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
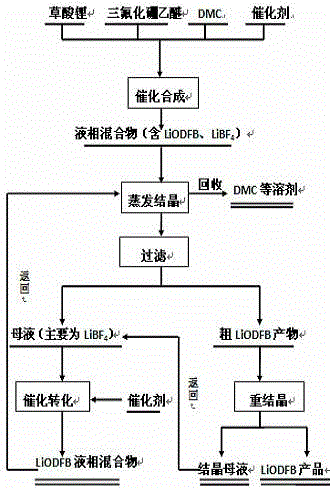
[0025] Add 300ml of dimethyl carbonate (DMC) into the reaction kettle, add 10g of lithium oxalate, 12.38ml of boron trifluoride ether at the same time, mix well, then slowly add 1ml of boron trichloride (BCl3), react at 40°C for 12h, A liquid phase mixture is obtained. Then vacuum distillation, 40 ° C, distillation for 1 h, and then at -20 ° C, insulation for 1 h to precipitate crystals. The crude product of lithium oxalate difluoroborate (LiODFB) was obtained by filtration, and the crude product was recrystallized to obtain a high-purity lithium oxalate difluoroborate (LiODFB) product. At the same time, collect the filtered mother liquor and recrystallized mother liquor, add 5.6g oxalic acid and 0.4g aluminum trichloride (AlCl3), react at 40°C for 12h, and return the obtained liquid phase mixture to the evaporation crystallization step. Finally, the lithium oxalate difluoroborate (LiODFB) solid obtained after drying at 40°C for 1 h has a purity of 99.9% and a yield of 90%. ...
Embodiment 2
[0027] Add 100ml of acetonitrile (AN) into the reaction kettle, add 10g of lithium oxalate, 37.5ml of boron trifluoride ether at the same time, mix well, then slowly add 10g of aluminum trichloride (AlCl3), react at 100°C for 24h, and obtain a liquid phase mixture. Then vacuum distillation, 80 ° C, distillation 5h, and then at 10 ° C, insulation 12h to precipitate crystals. The crude product of lithium oxalate difluoroborate (LiODFB) was obtained by filtration, and the crude product was recrystallized to obtain a high-purity lithium oxalate difluoroborate (LiODFB) product. At the same time, collect the filtered mother liquor and recrystallized mother liquor, add 28.2g oxalic acid, 0.32mL silicon tetrachloride (SiCl4), react at 100°C for 24h, and return the obtained liquid phase mixture to the evaporation crystallization step. Finally, the lithium oxalate difluoroborate (LiODFB) solid obtained after drying at 150°C for 48 hours had a purity of 99.9% and a yield of 92%.
Embodiment 3
[0029] Add 500ml of ethyl methyl carbonate (EMC) into the reaction kettle, add 10g of lithium oxalate, 24.76ml of boron trifluoride ether at the same time, mix well, then slowly add 5mL of silicon tetrabromide (SiBr4), react at 70°C for 18h, A liquid phase mixture is obtained. Then vacuum distillation, 60 ℃, distillation 3h, and then at -5 ℃, insulation 6.5h to precipitate crystals. The crude product of lithium oxalate difluoroborate (LiODFB) was obtained by filtration, and the crude product was recrystallized to obtain a high-purity lithium oxalate difluoroborate (LiODFB) product. At the same time, collect the filtered mother liquor and recrystallized mother liquor, add 16.9g oxalic acid and 1.6g aluminum trichloride (AlCl3), react at 70°C for 18h, and return the obtained liquid phase mixture to the evaporation crystallization step. Finally, the lithium oxalate difluoroborate (LiODFB) solid was obtained after drying at 95°C for 24.5 hours, with a purity of 99.9% and a yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com