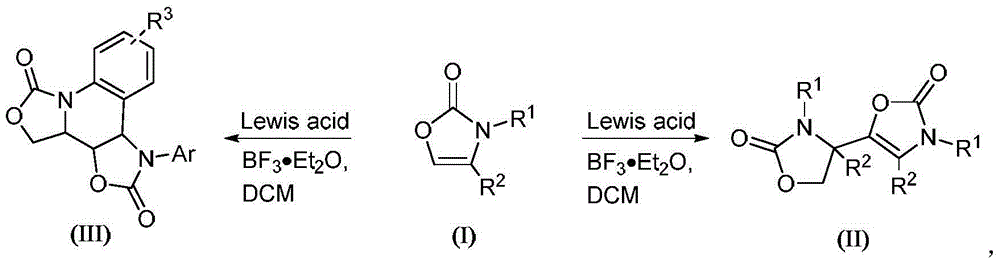
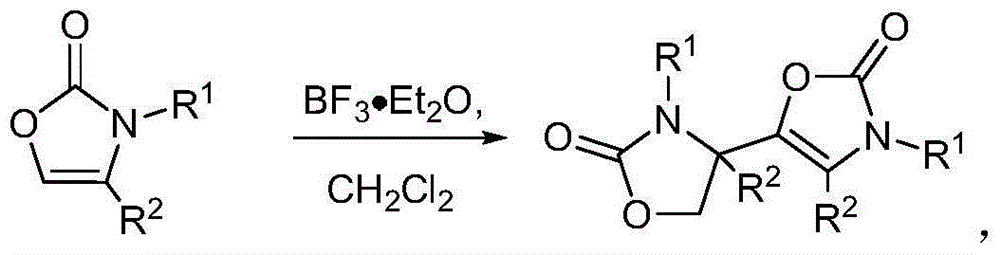Preparation method of N-substituted oxazolone polymer derivatives
A technology for oxazolones and derivatives, applied in the field of N-substituted oxazolones polymeric derivatives and preparation, can solve problems such as long reaction time, and achieve the effect of mild and efficient reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: 3-allyl-5-(3-allyl-2-oxooxazolidin-4-yl)oxazol-2(3H)-one
[0037]
[0038] In the DCM solution (2ml) of reaction substrate 1a (77.6mg, 0.62mmol), add BF dropwise 3 ·Et 2 O (41 μL, 0.5 equiv.), seal the tube. The reaction mixture was stirred at 50°C for 12 hours, and after the completion of the reaction was monitored with a TLC plate, it was washed with saturated NaHCO 3 (5ml) to quench the reaction and stir for 5 minutes. DCM was extracted 3 times (5mL x 3), the organic phases were combined and washed with water (10mL), saturated NaCl (10mL), and finally with NaCl 2 SO 4 After drying, filtration and rotary evaporation, the crude product was obtained. The crude product was subjected to silica gel column chromatography to obtain pure product 2a (yield 98%).
[0039] Colorless oily liquid; Rf=0.21 (Hexane / EtOAc=3 / 2); 1 H NMR (CDCl 3 ,400MHz):δ6.51(1H,s),5.88-5.82(1H,m),5.76-5.70(1H,m),5.34-5.15(4H,m),4.61(1H,dd,J=9.2, 7.2Hz), 4.60(1H,t,J=8.8Hz), 4.31...
Embodiment 2
[0040] Example 2: 5-(2-oxo-3-propyloxazolidin-4-yl)-3-propyloxazol-2(3H)-one
[0041]
[0042] In DCM solution (2ml) of reaction substrate 1b (79mg, 0.62mmol), add BF dropwise 3 ·Et 2 O (41 μL, 0.5 equiv.), seal the tube. The reaction mixture was stirred at 50°C for 12 hours, and after the completion of the reaction was monitored with a TLC plate, it was washed with saturated NaHCO 3 (5ml) to quench the reaction and stir for 5 minutes. DCM was extracted 3 times (5mL x 3), the organic phases were combined and washed with water (10mL), saturated NaCl (10mL), and finally with NaCl 2 SO 4 After drying, filtration and rotary evaporation, the crude product was obtained. The crude product was subjected to silica gel column chromatography to obtain pure product 2b (yield 85%).
[0043] Colorless oily liquid; Rf=0.22 (Hexane / EtOAc=3 / 2); 1 H NMR (CDCl 3 ,600MHz): δ6.54(1H,s),4.61(1H,dd,J=8.4,6.6Hz),4.45(1H,t,J=9.3Hz),4.30(1H,dd,J=8.4,6.6 Hz),3.55-3.52(2H,m),3.39-3.34(1H,m),3...
Embodiment 3
[0044] Example 3: 3-Benzyl-5-(3-benzyl-2-oxooxazolidin-4-yl)oxazol-2(3H)-one
[0045]
[0046] In the DCM solution (2ml) of reaction substrate 1c (109mg, 0.62mmol), add BF dropwise 3 ·Et 2 O (41 μL, 0.5 equiv.), seal the tube. The reaction mixture was stirred at 50°C for 12 hours, and after the completion of the reaction was monitored with a TLC plate, it was washed with saturated NaHCO 3 (5ml) to quench the reaction and stir for 5 minutes. DCM was extracted 3 times (5mL x 3), the organic phases were combined and washed with water (10mL), saturated NaCl (10mL), and finally with NaCl 2 SO 4 After drying, filtration and rotary evaporation, the crude product was obtained. The crude product was subjected to silica gel column chromatography to obtain pure product 2c (yield 87%).
[0047] White solid (melting point: 125-126 °C); Rf = 0.33 (Hexane / EtOAc = 3 / 2); 1 H NMR (CDCl3, 400MHz): δ7.40-7.14 (10H, m), 6.23 (1H, s), 4.69 (1H, d, J=15.6Hz), 4.66 (2H, s), 4.38-4.29 (3H ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com