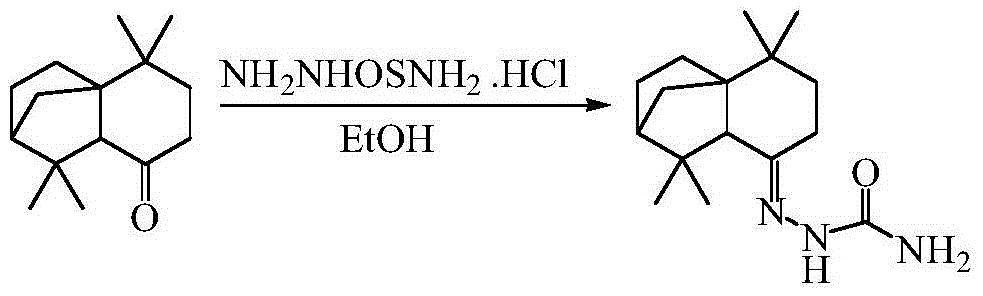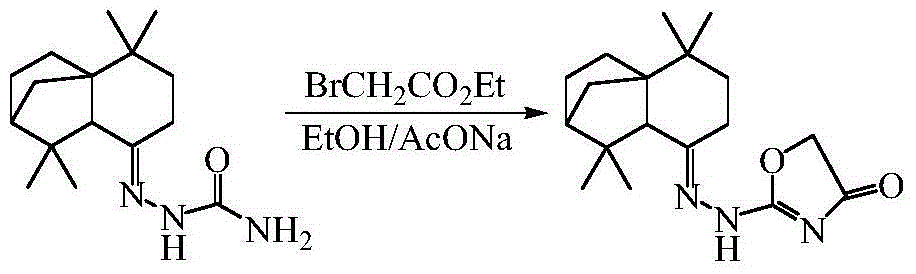Isolongifolene alkyl oxazolone as well as synthesis method and application thereof
A technology of alkyl oxazolone and synthesis method, applied in the direction of drug combination, organic chemistry, non-central analgesics, etc., to achieve the effect of expanding the field of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthetic method of isolongifolyl oxazolone, the steps are as follows:
[0025] 1) Condensation and substitution reaction between isolonganone and semicarbazide hydrochloride to generate semicarbazone.
[0026]
[0027] The specific operation is: in a 50mL three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, add isolonganone (2mmol), semicarbazide hydrochloride (2mmol), and absolute ethanol 20mL in sequence, and heat and stir. After heating to reflux temperature, add an appropriate amount of concentrated sulfuric acid catalyst, and react for 12-24 hours (monitored by LC-MS). Continuously washed with sodium chloride for 5 times, dried over anhydrous sodium sulfate, filtered, rotary evaporated, recrystallized with 95% ethanol, and purified to obtain isolongifolyl semicarbazone.
[0028] 2) Cyclization reaction of semicarbazone compounds with ethyl bromoacetate (or ethyl chloroacetate) to generate corresponding thiazolone compo...
Embodiment 2
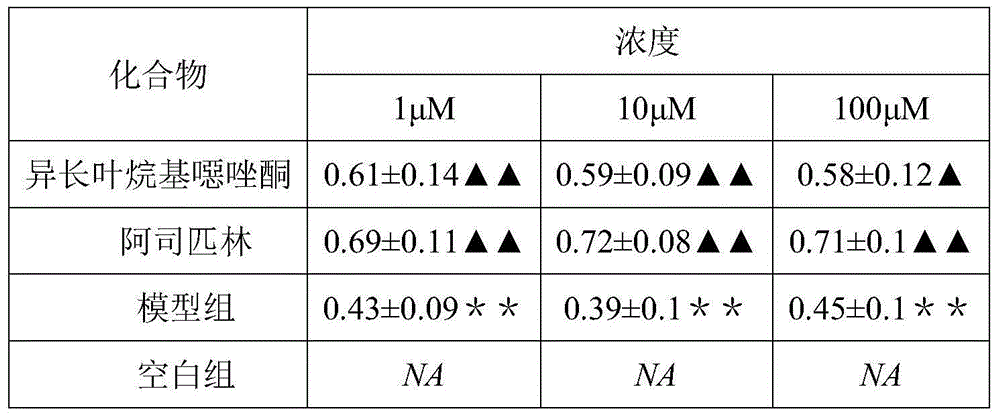
[0034] Inhibitory activity test of isolongifolyl oxazolones on the inflammatory response of human umbilical vein endothelial cells (HUVECs).
[0035] 1. Cell culture method: culture human umbilical vein endothelial cells in DMEM medium with 10% calf serum. Put in CO 2 in an incubator (37°C, 5% CO 2 , 95% air, maintain a certain humidity environment), and observe the growth of cells under an inverted microscope. According to the actual condition of the cells, change the medium; after 1-2 days of culture, the cells can become a monolayer, and then digested with 0.25% trypsin, and passaged at a ratio of 1:3. Human umbilical vein endothelial cells grown as monolayers were used during the experiments.
[0036] 2. Establishment and grouping of experimental models: First, cells were digested with 0.25% trypsin, and DMEM medium containing 10% calf serum was added. Use a dropper to blow into a single cell suspension, and inoculate the cells in a 96-well culture plate at a seeding d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com