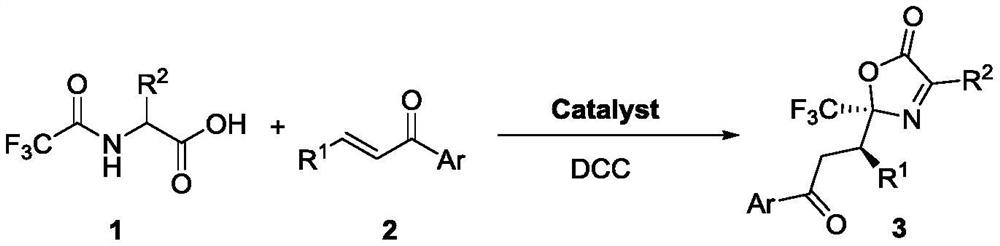
Method for synthesizing trifluoromethyl-containing oxazolone compound by one-pot method
A technology of trifluoromethyl oxazolone and compounds, which is applied in the field of asymmetric synthesis, and can solve problems affecting yield and ee value, difficult to control, easy to deteriorate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] The synthetic steps of racemic compound 3 are as follows:
Embodiment 2
[0030]
[0031]
[0032]
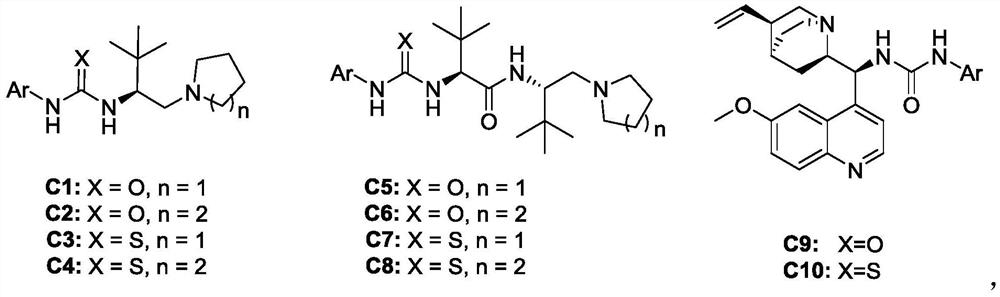
[0033] a Unless otherwise stated, la (0.075 mmol), Cat. (0.005 mmol), 2a (0.05 mmol), solvent (0.5 mL). b Isolated yield. c The dr value was obtained by proton spectrum analysis of the crude product. d The ee value is obtained by chiral column HPLC chiral analysis. EDCI: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, EDC: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide, DCC : N,N'-dicyclohexylcarbodiimide.
[0034] In the screening process of reaction conditions, the influence of different chiral catalysts on the reaction was first investigated (entries1-10), and it was found that C10 was the best chiral catalyst. In addition, in addition to the catalysts listed in the above table, there are many catalysts used Products with different ee values can also be obtained, such as amino acid derivatives, quinines, quinidines and other alkaloid catalysts. Afterwards, the effects of different condensing agents on the reaction were ...
Embodiment 3
[0038]
[0039] Compound 1a (0.15mmol, 1.5eq), catalyst (0.01mmol, 0.1eq), DCC (0.15mmol, 1.5eq), 1mL mesitylene and compound 2b (0.1mmol, 1.0eq) were added to the reaction flask at 25 The reaction was carried out under the condition of ℃, and the reaction was monitored by thin-layer plate (TLC). After 2 hours of reaction, column chromatography (eluent: PE:EA=50:1-10:1) separated and obtained white solid 3b, 91% ee, product The rate is 90%. 1 H NMR (400MHz, CDCl 3 )δ8.07(d,J=8.2Hz,2H),7.76(d,J=8.2Hz,2H),4.21-4.06(m,2H),4.04(dd,J=10.3,3.5Hz,1H), 3.73(dd,J=18.3,10.3Hz,1H),3.57(dd,J=18.3,3.5Hz,1H),1.37(s,9H),1.28(t,J=7.1Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com