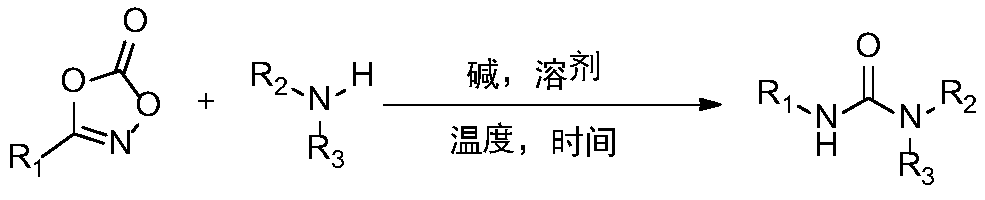
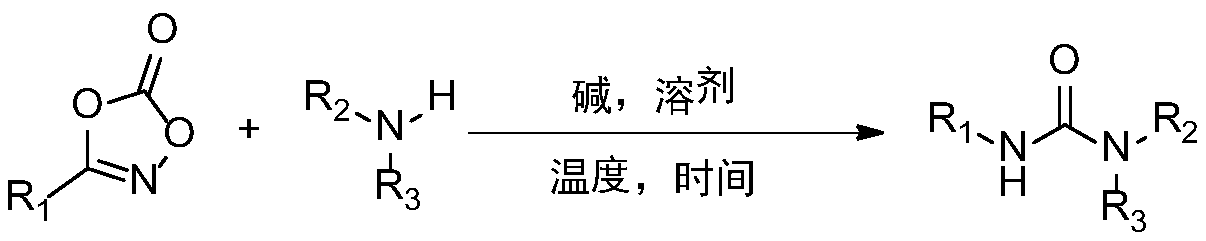
Synthetic method and application of urea compound
A synthesis method and compound technology, which is applied in the field of synthesis of urea compounds, can solve the problems of using unstable starting materials and being expensive, and achieve the effects of low cost, easy raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
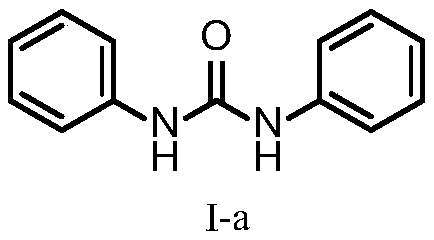
[0018] Example 1 A method for preparing urea compounds, preparing the compound represented by formula I-a
[0019]
[0020] The preparation steps are as follows: In the air, add magneton, 3-phenyl-1,4,2-oxazol-5-one (0.3mmol), sodium acetate (0.3mmol) and a 10mL round bottom flask. Methanol (1 mL), add aniline (0.3 mmol) under stirring. Then plug the rubber stopper and heat and stir in an oil bath at 60°C for 2h. After the reaction is completed, petroleum ether / ethyl acetate is selected as the mobile phase, and the product is purified by flash silica gel column chromatography.
[0021] The yield was 99%. The structure of the compound represented by formula I-a is characterized as follows: white solid, 1 H NMR (500MHz, DMSO): δ8.66 (2H, s), 7.46 (4H, dd, J = 8.5, 1.0 Hz), 7.28 (4H, td, J = 7.5, 1.5 Hz), 6.96 (4H, tt ,J=7.5,1.0Hz); 13 C NMR(125MHz,DMSO):δ152.5,139.7,128.8,121.8,118.2; HRMS(ESI)m / z calcd for C 13 H 13 N 2 O[M+H] + 213.1028,found 213.1038.
Embodiment 2
[0022] Example 2 A method for preparing urea compounds, using two methods to prepare the compound represented by formula I-b
[0023]
[0024] Method 1 The preparation steps are as follows: In the air, add magneton, substituted oxazolone (0.3mmol), sodium acetate (0.3mmol) and methanol (1mL) into a 10mL round bottom flask, and add aniline under stirring. (0.3mmol). Then plug the rubber stopper and heat and stir in an oil bath at 60°C for 2h. After the reaction is completed, petroleum ether / ethyl acetate is selected as the mobile phase, and the product is purified by flash silica gel column chromatography.
[0025] Method 2 The preparation steps are as follows: In the air, add magneton, 3-phenyl-1,4,2-dioxazol-5-one (0.3mmol), sodium acetate (0.3mmol) to a 10mL round bottom flask. mmol) and methanol (1 mL), and add substituted amine (0.3 mmol) under stirring. Then plug the rubber stopper and heat and stir in an oil bath at 60°C for 2h. After the reaction is completed, petroleum ...
Embodiment 3
[0027] Example 3 A method for preparing urea compounds, using two methods to prepare the compound represented by formula I-c
[0028]
[0029] Method 1 The preparation steps are as follows: In the air, add magneton, substituted oxazolone (0.3mmol), sodium acetate (0.3mmol) and methanol (1mL) into a 10mL round bottom flask, and add aniline under stirring. (0.3mmol). Then plug the rubber stopper and heat and stir in an oil bath at 60°C for 2h. After the reaction is completed, petroleum ether / ethyl acetate is selected as the mobile phase, and the product is purified by flash silica gel column chromatography.
[0030] Method 2 The preparation steps are as follows: In the air, add magneton, 3-phenyl-1,4,2-dioxazol-5-one (0.3mmol), sodium acetate (0.3mmol) to a 10mL round bottom flask. mmol) and methanol (1 mL), and add substituted amine (0.3 mmol) under stirring. Then plug the rubber stopper and heat and stir in an oil bath at 60°C for 2h. After the reaction is completed, petroleum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com