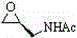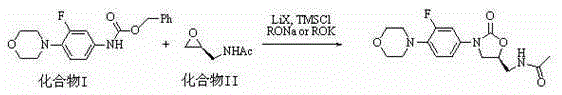Preparation method for Linezolid
The technology of linezolid and compound is applied in the field of preparation of oxazolidinone antibiotics, can solve the problems of easy moisture absorption, difficult to store, uneconomical atom/process, etc., and achieves mild reaction conditions, high yield and purity, The effect of easy availability of reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
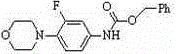
[0026] Under the protection of nitrogen, add 0.33g (1.0mmol) compound I, 0.05g (1.2mmol) lithium chloride, 0.14g (1.5mmol) sodium tert-butoxide, 0.13g (1.2mmol) trimethylchlorosilane (1.2mmol) to 20mL dry tetrahydrofuran ), stirred at room temperature for 40 minutes, cooled to 0°C, added dropwise 0.28g (2.5mmol) of compound II dissolved in 10mL of tetrahydrofuran, stirred at 0°C for 30 minutes, then raised the temperature to 28°C for 12 hours, HPLC showed that 85% of the raw compound I had reacted completely. Add acetic acid to neutralize to a pH value of 6-7, then add 50mL dichloromethane and 30mL water solution, extract the water phase with 3×30mL dichloromethane, combine the organic phases with anhydrous sodium sulfate, filter and concentrate, and the oily product is washed with acetic acid Ethyl ester was recrystallized, cooled to room temperature and then crystallized at -15°C, filtered, washed with glacial ethyl acetate, and vacuum dried to obtain 0.18 g of white powdery...
Embodiment 2
[0028] Under nitrogen protection, add 0.33g (1.0mmol) of compound I, 0.07g (0.8mmol) of lithium bromide, 0.16g (1.3mmol) of potassium tert-amylate, and 0.11g of trimethylformamide to 15mL of dry N,N-dimethylformamide Chlorosilane (1.1mmol), stirred at room temperature for 40min, cooled to 0°C, 0.21g (1.8mmol) of compound II dissolved in 5mL N,N-dimethylformamide was added dropwise, stirred at 0°C for 30min, then heated to 30 React at ℃ for 10 hours, add saturated ammonium chloride aqueous solution to neutralize to pH 6-7, then add 50mL chloroform and 30mL water solution, extract the water phase with 3×30mL chloroform, combine the organic phases and dry them with anhydrous sodium sulfate, filter and concentrate. The oil was recrystallized with acetone, cooled to room temperature and then crystallized at -15°C, filtered, washed with ice acetone, and vacuum dried to obtain 0.15 g of a white powdery solid with a purity of >96% and a yield of 47%.
Embodiment 3
[0030] Under the protection of argon, add 0.33g (1.0mmol) compound I, 0.04g (1.0mmol) lithium chloride, 0.28g (2.5mmol) potassium n-butoxide, 0.15g trimethylchlorosilane (1.4 mmol), stirred at room temperature for 40 minutes, cooled to 0°C, added dropwise 0.32 g (3.0 mmol) of compound II dissolved in 5 mL of acetonitrile, and stirred at 0°C for 40 minutes, then raised the temperature to 30°C for 22 hours, added saturated ammonium chloride aqueous solution to neutralize To pH 6-7, add 50mL chloroform and 30mL water solution, extract the water phase with 3×30mL chloroform, combine the organic phase and dry it with anhydrous sodium sulfate, filter, concentrate, recrystallize the oil with butyl acetate, and cool to room temperature Then crystallize at -15°C, filter, wash the filter cake with glacial butyl acetate, and vacuum dry to obtain 0.20 g of white powdery solid with a purity of >96% and a yield of 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com