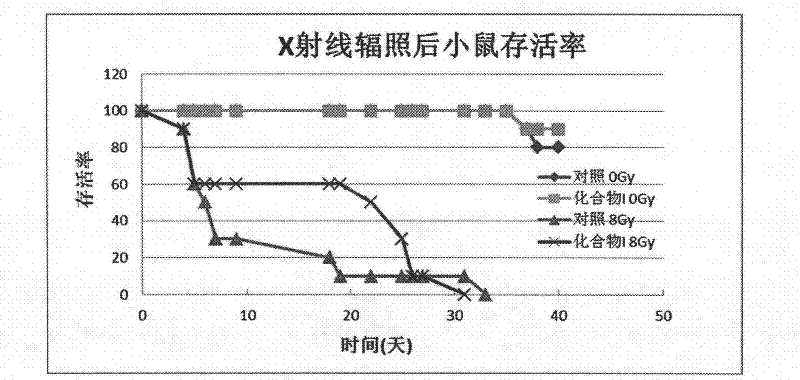
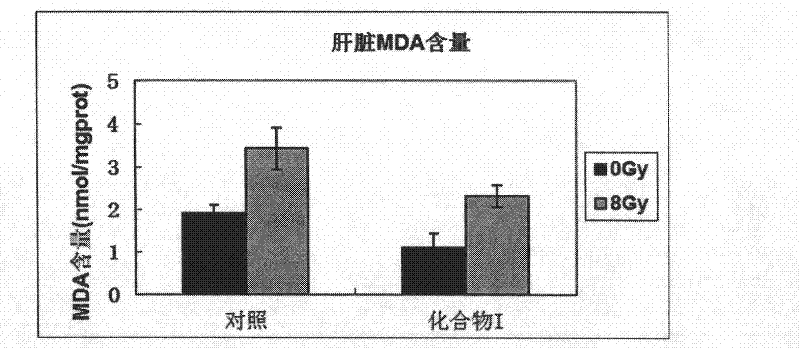
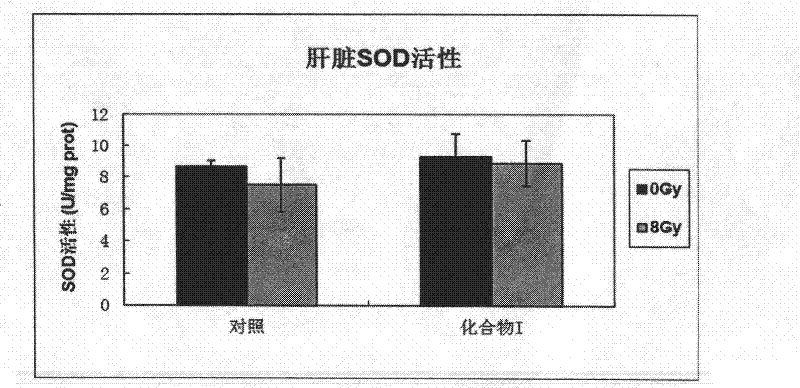
Anti-radiation medicine and manufacturing method and application
An anti-radiation and drug technology, applied in the field of medicine, can solve the problems of unreported anti-radiation effects of compounds, and achieve the effects of improving relative survival rate, reducing DNA damage, and reducing instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Synthesis of 4-(4-methoxybenzylidene)-2-phenyl-5(4H)-oxazolone (I)
[0043] Under nitrogen flow, into a three-neck flask (100 mL) that was fully dried and replaced with nitrogen, with a dropping funnel, condenser, and thermometer, was added hippuric acid (1.79 g, 10 mmol), NaHCO 3(0.40g, 4.0mmol), p-methoxybenzaldehyde (1.36g, 10mmol), slowly drop into acetic anhydride (10mL), the mixture was heated to 120°C, and kept stirring for 4h. After the reaction was completed, it was left to cool to room temperature, and the reactant was poured into water (100 mL) to generate a yellow target solid, which was filtered off. The crude product was washed twice with hot water (90°C, 10 mL), then washed twice with cold ethanol (0°C, 5 mL), and dried under vacuum at room temperature, thus obtaining 4-(4-methoxybenzylidene )-2-phenyl-5(4H)-oxazolone 2.24g.
[0044] The structure of the compound is determined by nuclear magnetic resonance and mass spectrometry, and the yield...
Embodiment 2
[0047] Embodiment 2: Adopt the same method of embodiment 1, can obtain:
[0048] 4-(benzylidene)-2-phenyl-5(4H)-oxazolone (II);
[0049] 4-(4-Bromobenzylidene)-2-phenyl-5(4H)-oxazolone (III);
[0050] 4-(4-Acetoxybenzylidene)-2-phenyl-5(4H)-oxazolone (IV);
[0051] 4-(4-methoxy-3-methoxybenzylidene)-2-phenyl-5(4H)-oxazolone (V);
[0052] 4-(4-Methylbenzylidene)-2-phenyl-5(4H)-oxazolone (VI);
[0053] 4-(4-nitrobenzylidene)-2-phenyl-5(4H)-oxazolone (VII);
[0054] 4-(3,4-dimethoxybenzylidene)-2-phenyl-5(4H)-oxazolone (VIII);
[0055] 4-(4-N,N-Dimethylbenzylidene)-2-phenyl-5(4H)-oxazolone (IX)
Embodiment 3
[0056] Example 3: Synthesis of 4-(4-methoxybenzylidene)-2-(p-methoxyphenyl)-5(4H)-oxazolone (X)
[0057] Under a stream of nitrogen, to a three-neck flask (100 mL) that was fully dried and replaced with nitrogen, with a dropping funnel, a condenser, and a thermometer, was added (4-methoxy)benzoyl-glycine (2.10 g, 10 mmol) , NaHCO 3 (0.40g, 4.0mmol), p-methoxybenzaldehyde (1.36g, 10mmol), slowly drop into acetic anhydride (10mL), the mixture was heated to 120°C, and kept stirring at the temperature for 4 hours. After the reaction was completed, it was left to cool to room temperature, and the reactant was poured into water (100 mL) to generate a yellow target solid, which was filtered off. The crude product was washed twice with hot water (90°C, 10 mL), then washed twice with cold ethanol (0°C, 5 mL), and dried under vacuum at room temperature, thus obtaining 4-(4-methoxybenzylidene )-2-(p-methoxyphenyl)-5(4H)-oxazolone.
[0058] The structure of the compound is determined b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com