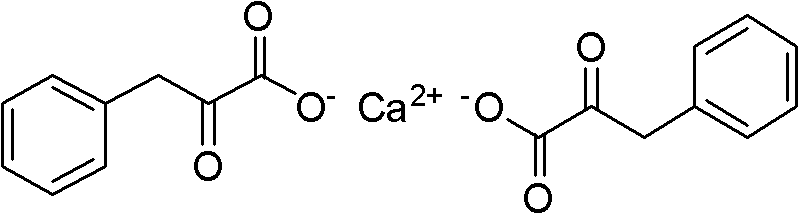Method for preparing Alpha-keto-phenylalanine calcium
A technology of calcium ketophenylalanine and glycine is applied in the field of preparation of calcium α-ketophenylalanine, and can solve the problems of difficult hydrolysis of 5-benzylidene hydantoin, long reaction time, low hydrolysis yield and the like , to achieve the effect of easy reaction control, small equipment investment and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
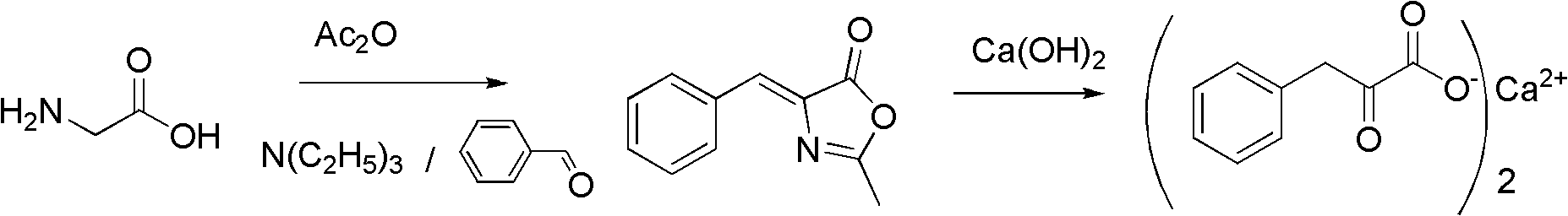
[0044] Embodiment 1: Preparation of 4-benzylidene-2-methyldihydrooxazolone
[0045] Put 37.5g (0.5mol) of pharmaceutical grade glycine and 50.5g (0.5mol) of triethylamine into a 500mL three-neck flask equipped with mechanical stirring, thermometer, and reflux condenser, stir evenly, and add dropwise under stirring and cooling control at 50°C 127.5g (1.25mol) of acetic anhydride, after the addition of acetic anhydride is completed, stir and react at 40-50°C for 1 hour, then add 53g (0.5mol) of benzaldehyde dropwise in 15 minutes, and raise the temperature to 100-120°C to continue the reaction 1-2 hours until the benzaldehyde peak disappears, the reaction is complete, the mixture of acetic anhydride, acetic acid, and triethylamine is distilled off under reduced pressure, and can be used in the next batch of reactions after rectification, and the residue is cooled to become a solid 4-methanol. Benzyl-2-methyldihydrooxazolone, weight 88.6g, content 95.5%, yield 90.4%. This conten...
Embodiment 2-6
[0046] Example 2-6: Preparation of 4-benzylidene-2-methyldihydrooxazolone under the same conditions as in Example 1 except that different catalysts were used.
[0047]
Embodiment 7
[0048] Example 7: Preparation of 4-benzylidene-2-methyldihydrooxazolone
[0049] In a 500mL three-neck flask equipped with a mechanical stirrer, a thermometer, and a reflux condenser, put 37.5g (0.5mol) of pharmaceutical grade glycine and 33.3g (0.5mol) of N,N'-dimethylpiperazine, stir evenly, and stir to cool Add 127.5g (1.25mol) of acetic anhydride dropwise under control at 50°C. After the dropwise addition of acetic anhydride is complete, stir and react at 40-50°C for 1 hour, then add 53g (0.5mol) of benzaldehyde dropwise in 15 minutes, and raise the temperature after dropping Continue to react at 100-120°C for 1-2 hours until the peak of benzaldehyde disappears. After the reaction is complete, distill the mixture of acetic anhydride and acetic acid under reduced pressure. Evaporate to dryness under reduced pressure, and cool to obtain a solid of 4-benzylidene-2-methyldihydrooxazolone, with a weight of 88.9 g, a content of 97.5%, and a yield of 92.6%. This content of 4-ben...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com