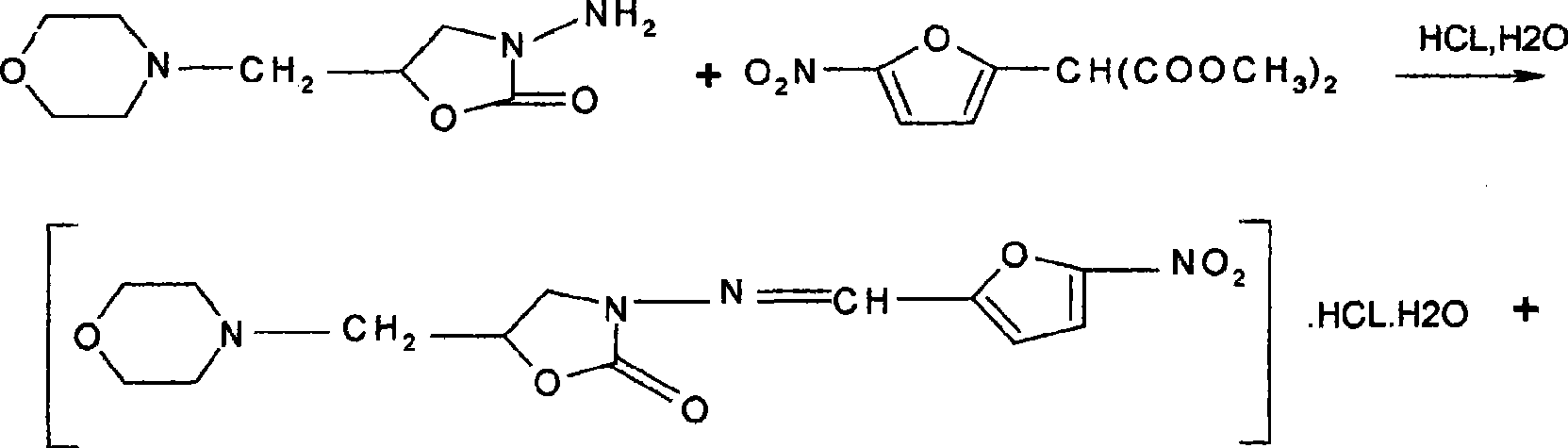
Process for preparing furaltadone hydrochloride
A kind of furaltadone hydrochloride and technology, applied in the field of organic synthesis, can solve the problems of low yield, many reaction steps, troublesome operation and the like, and achieve the effects of high yield and simplified operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0007] (1) Put 500L water and 200kg concentration of 31% hydrochloric acid into the tank, cover the feeding port, and open the condenser cooling water; (2) Then the reaction tank jacket enters the steam, and when the temperature rises to 46°C, add 55kg5-nitrate Diethylfurfural diethyl ester, slowly heat up, 15°C per hour, when the temperature rises to 61°C, add 95kg morpholine methyleneamino azlactone (3) and continue to slowly heat up, when the temperature in the tank rises to 80°C, maintain 80--85°C temperature for 30 minutes; (4) Stop the steam after heat preservation, press filter, put the filtrate into the crystallization tank, start stirring, and cool down to 10°C; (5) Centrifuge to dry, wash with 20 kg of 95% ethanol Shake dry.
Embodiment 2
[0009] (1) Put 500L water and 200kg concentration of 31% hydrochloric acid into the tank, cover the feeding port, and open the condenser cooling water; (2) Then the reaction tank jacket enters the steam, and when the temperature rises to 48°C, add 55kg5-nitrate Diethylfurfural diethyl ester, slowly heat up, 15°C per hour, when the temperature rises to 63°C, add 95kg morpholine methyleneamino azlactone (3) and continue to slowly heat up, when the temperature in the tank rises to 80°C, maintain 80--85°C temperature for 30 minutes; (4) Stop the steam after heat preservation, press filter, put the filtrate into the crystallization tank, start stirring, and cool down to 10°C; (5) Centrifuge to dry, wash with 20 kg of 95% ethanol Shake dry.
Embodiment 3
[0011] (1) 500L water and 200kg concentration of 31% hydrochloric acid are put into the tank, cover the feeding port, and open the condenser cooling water; (2) Then the reaction tank jacket enters steam, and when the temperature is raised to 50°C, add 55kg 5- Nitrofurfural diethyl ester slowly heats up to 15°C per hour. When the temperature rises to 65°C, add 95kg morpholine methyleneamino azlactone (3) and continue to slowly heat up. When the temperature in the tank rises to 80°C, Maintain the temperature at 80--85°C for 30 minutes; (4) stop the steam after heat preservation, press filter, put the filtrate into the crystallization tank, start stirring, and cool down to 10°C; (5) spin dry in a centrifuge, wash with 20 kg of 95% ethanol Shake dry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com