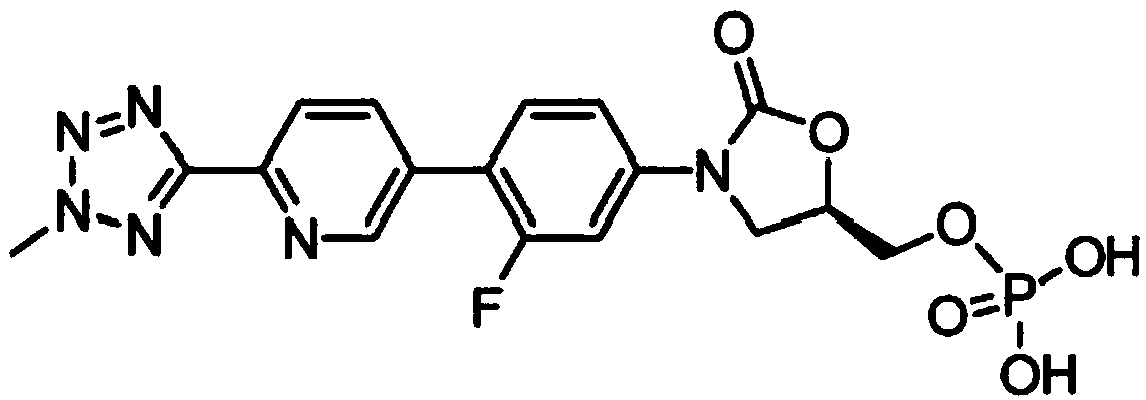
Preparation method of tedizolid phosphate
A technology of tedizolid phosphate and compound, applied in the field of preparation of tedizolid phosphate, can solve the problems of high price, impurity and high cost, and achieve the effects of reducing catalyst cost, mild reaction conditions and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
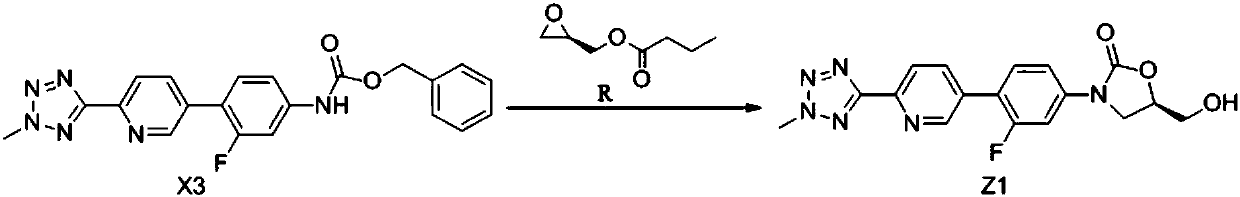
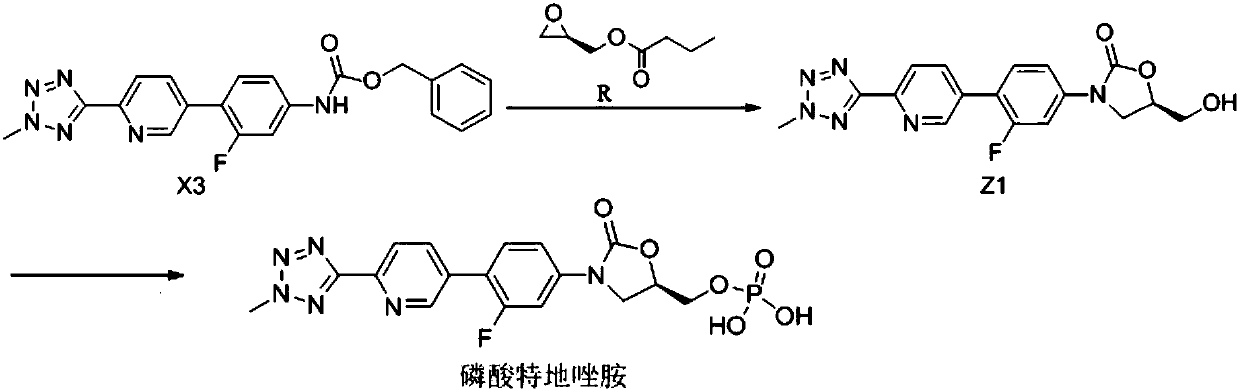
[0057] The preparation method of the compound shown in embodiment 1 formula Z1
[0058] Add 50g of the compound of formula X3, 400ml of tetrahydrofuran, and 400ml of acetonitrile into a 1L three-necked flask, blow nitrogen into it, control the temperature at 25°C, the solid is insoluble, then add 19.8g of lithium tert-butoxide, the solid is dissolved, and the reaction solution changes from colorless to Yellow color, after stirring for 2 hours, add 19.7 g of R-(-)-glycidyl butyric acid and the compound of formula R dropwise to the reaction solution, keep warm for 3 hours after the dropwise addition, and take a sample for TLC (developer: chloroform / Methanol = 10 / 1), when the spots of the compound of formula X3 disappear, add dropwise dilute hydrochloric acid prepared from concentrated hydrochloric acid and water, adjust the pH to 8, and stir thoroughly for 30 minutes. Concentrate under reduced pressure at 0.07MPa~-0.1MPa to cut off the flow to obtain the compound represented by...
Embodiment 2
[0059] The preparation method of the compound shown in embodiment 2 formula Z1
[0060] Add 50g of the compound of formula X3, 400ml of tetrahydrofuran, and 400ml of acetonitrile into a 1L three-necked flask, blow nitrogen into it, control the temperature at 25°C, the solid is insoluble, then add 23.8g of sodium tert-butoxide, the solid is dissolved, and the reaction solution changes from colorless to Yellow color, after stirring for 2 hours, add 19.7 g of R-(-)-glycidyl butyric acid and the compound of formula R dropwise to the reaction solution, keep warm for 3 hours after the dropwise addition, and take a sample for TLC (developer: chloroform / Methanol = 10 / 1), when the spots of the compound of formula X3 disappear, add dropwise dilute hydrochloric acid prepared from concentrated hydrochloric acid and water, adjust the pH to 8, and stir thoroughly for 30 minutes. Concentrate under reduced pressure at 0.07MPa~-0.1MPa to cut off the flow to obtain the compound represented by ...
Embodiment 3
[0061] The preparation method of the compound shown in embodiment 3 formula Z1
[0062] Add 50g of the compound of formula X3, 400ml of tetrahydrofuran, and 400ml of acetonitrile into a 1L three-necked flask, blow nitrogen into it, control the temperature at 25°C, the solid is insoluble, then add 27.8g of potassium tert-butoxide, the solid dissolves, and the reaction solution changes from colorless to Yellow color, after stirring for 2 hours, add 19.7 g of R-(-)-glycidyl butyric acid and the compound of formula R dropwise to the reaction solution, keep warm for 3 hours after the dropwise addition, and take a sample for TLC (developer: chloroform / Methanol = 10 / 1), when the spots of the compound of formula X3 disappear, add dropwise dilute hydrochloric acid prepared from concentrated hydrochloric acid and water, adjust the pH to 8, and stir thoroughly for 30 minutes. Concentrate under reduced pressure at 0.07MPa~-0.1MPa to cut off the flow to obtain the compound represented by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com