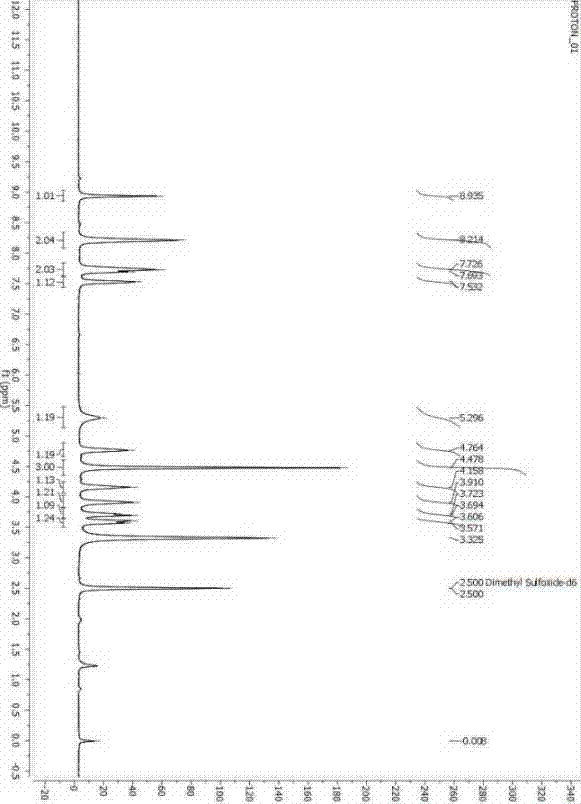
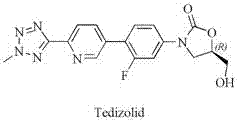
One-pot synthesized tedizolid
A technology of tedizolid and tetrazole, applied in the field of preparation of tedizolid, can solve problems such as unfavorable scale-up production, harsh reaction conditions, complicated operation process, etc., and achieves reduction of material transfer, processing cost, and solvent reduction. effect of dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Under stirring, 1,4-dioxane (300 ml), 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine (20.0 g, 83.3 mmol , 1.0 equiv), pinacol diboronate (21.2 g, 83.3 mmol, 1.0 equiv), potassium acetate (24.5 g, 250 mmol, 3.0 equiv), and tetrakis(triphenylphosphine)palladium (2.0 g, 1.7 mmol, 0.02 equivalent). The temperature was raised to reflux under the protection of nitrogen, and the reaction of 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine was followed by TLC to complete the reaction.
[0033] After the reaction is completed, cool to below 10°C, and add (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one (24.1 g, 83.3 mmol, 1.0 equivalent), potassium carbonate (34.5 g, 250 mmol, 3.0 equivalent) and tetrakis(triphenylphosphine)palladium (0.96 g, 0.83 mmol, 0.01 equivalent), nitrogen protection. Warming up to reflux, TLC tracking 2-(2-methyl-2H-tetrazol-5-yl)-5-(4,4,5,5-tetramethyl-1,3-dioxaborolane-2- Base) pyridine reacts completely.
[0034] After the reaction was ...
Embodiment 2
[0036] Under stirring, 1,4-dioxane (400 ml), 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine (20.0 g, 83.3 mmol , 1.0 equiv), pinacol diboronate (25.3 g, 100 mmol, 1.2 equiv), potassium acetate (20.4 g, 208 mmol, 2.5 equiv), and bis(triphenylphosphine)palladium dichloride (1.17 g, 1.4 mmol, 0.015 equiv). The temperature was raised to reflux under the protection of nitrogen, and the reaction of 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine was followed by TLC to complete the reaction.
[0037] After completion of the reaction, cool to below 20°C, add (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one (21.7 g, 75.0 mmol, 0.9 equiv), potassium carbonate (34.5 g, 250 mmol, 3.0 equiv), palladium acetate (0.14 g, 0.62 mmol, 0.0075 equiv) and triphenylphosphine (0.65 g, 2.5 mmol, 0.03 equiv), under nitrogen protection. Warming up to reflux, TLC tracking 2-(2-methyl-2H-tetrazol-5-yl)-5-(4,4,5,5-tetramethyl-1,3-dioxaborolane-2- Base) pyridine reacts completely.
[0038]A...
Embodiment 3
[0040] Under stirring, 1,4-dioxane (400 ml), 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine (20.0 g, 83.3 mmol , 1.0 equiv), pinacol diboronate (23.3 g, 91.6 mmol, 1.1 equiv), potassium phosphate (35.3 g, 167 mmol, 2.0 equiv) and [1,1'-bis(diphenylphosphino) di Ferrocene]palladium dichloride (0.61 g, 0.83 mmol, 0.01 equiv). The temperature was raised to reflux under the protection of nitrogen, and the reaction of 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine was followed by TLC to complete the reaction.
[0041] After the reaction is completed, cool to below 25 °C, add water (60 ml), (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazolidine-2- Ketone (26.5 g, 91.4 mmol, 1.1 equiv), cesium carbonate (54.3 g, 167 mmol, 2.0 equiv), palladium chloride (0.14 g, 0.62 mmol, 0.01 equiv) and triphenylphosphine (0.65 g, 2.5 mmol, 0.04 equivalent), nitrogen protection. Warming up to reflux, TLC tracking 2-(2-methyl-2H-tetrazol-5-yl)-5-(4,4,5,5-tetramethyl-1,3-dioxaborolane-2- Base) p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com