Method for preparing tedizolid phosphate
A technology of tedizolid phosphate and glycerin butyrate, which is applied in the field of preparation of tedizolid phosphate, can solve the problems of phosphate bond being easily broken and hydrolyzed, unstable yield and impurity spectrum, and harsh water requirements, so as to avoid breakage Hydrolysis, good reaction reproducibility, and the effect of reducing the cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
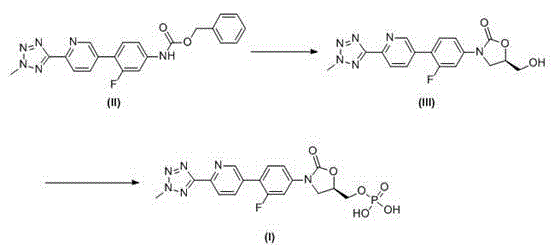
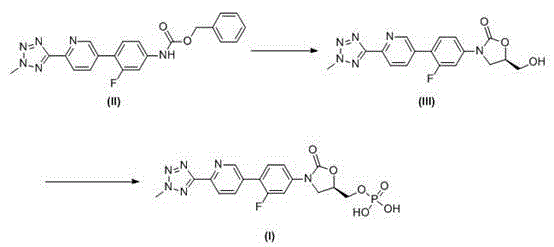
[0034]Example 1: (5R)-3-(4-(6-(2-methyl-2H-tetrazol-5-yl)-3-pyridyl)-3-fluorophenyl)-5-hydroxymethyl Oxazolin-2-one (III) preparation of
[0035] Option 1: Potassium tert-butoxide as base
[0036] Add tetrahydrofuran (20 L) and N-[3-fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridyl]phenyl]carbamate to the kettle Benzyl ester compound (II) (1 Kg, 2.47 mol), after stirring at 20~30°C, add 1,3-dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone (DMPU, 0.63 Kg, 4.94 mol, 2 times equivalent) and potassium tert-butoxide (0.33 Kg, 2.96 mol, 1.2 times equivalent), add R-glycidyl butyrate (0.39 Kg, 2.72 mol, 1.1 times equivalent), and react at 0~30°C for 15 hours. After the reaction was completed, the ammonium chloride solution (1 Kg / 10 L) with a mass-volume concentration of 10% was added to quench the reaction, concentrated under reduced pressure, and the residue was added to methanol (10 L), stirred for 0.5 hours to obtain suction filtration, The filter cake was washed with methanol a...
Embodiment 2
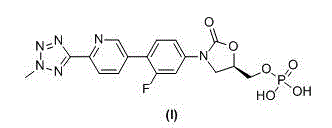
[0045] Example 2: (5R)-3-(4-(6-(2-methyl-2H-tetrazol-5-yl)-3-pyridyl)-3-fluorophenyl)-2-oxo-5 -oxazolidinyl) methyl phosphate (I) Crude preparation
[0046] Add tetrahydrofuran (30 L) and (5R)-3-(4-(6-(2-methyl-2H-tetrazol-5-yl)-3-pyridyl)-3-fluorobenzene to the reaction kettle base)-5-hydroxymethyloxazolin-2-one compound (III) (1.5 Kg, 4.05 mol), after stirring evenly, add triethylamine (1.23 Kg, 12.15 mol, 3 times of equivalents), drop phosphorus oxychloride (1.86 Kg, 12.15 mol , 3 times the equivalent) of tetrahydrofuran (12 L) solution, after the dropwise addition, continue to maintain the temperature at 0 ~ 15 ° C and stir for 2 hours, add purified water (25 L) to quench the reaction, stir at 0 ~ 15 ° C for 15 hours, Suction filtration, the filter cake was washed with purified water and acetone successively, and vacuum-dried at 45~50°C to obtain (5R)-3-(4-(6-(2-methyl-2H-tetrazole-5 -yl)-3-pyridyl)-3-fluorophenyl)-2-oxo-5-oxazolidinyl)methyl phosphate compound (I)...
Embodiment 3
[0047] Example 3: (5R)-3-(4-(6-(2-methyl-2H-tetrazol-5-yl)-3-pyridyl)-3-fluorophenyl)-2-oxo-5 -oxazolidinyl) methyl phosphate (I) preparation of
[0048] Option 1: The base is sodium bicarbonate
[0049] Add (5R)-3-(4-(6-(2-methyl-2H-tetrazol-5-yl)-3-pyridyl)-3-fluorophenyl)-2-oxo- 5-oxazolidinyl) methyl phosphate (I) Crude product (0.8 Kg), adjust the pH value of the reaction solution to 7~9 with a mass-volume concentration of 5% sodium bicarbonate solution (0.4 Kg / 8 L), heat to 40~45°C and stir for 0.5 hours, add acetone dropwise (16 L), cooled to 0 ~ 5 ° C after the dropwise addition, stirred for 20 minutes, suction filtered to obtain tedizolid phosphate sodium salt, dissolved in ethanol (16 L), heated to 40 ~ 45 ° C, Use 2 mol / L hydrochloric acid solution (4 L) to adjust the pH value of the reaction solution to 1~2, cool down to 0~5°C, filter with suction, wash the filter cake with purified water and acetone successively, and vacuum dry at 45~50°C (5R)-3-(4-(6-(2-Met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com