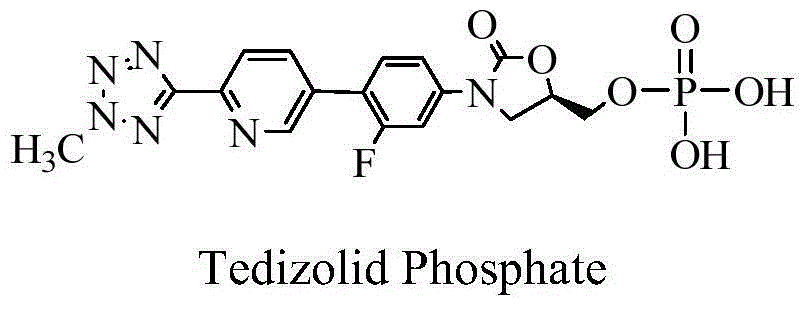
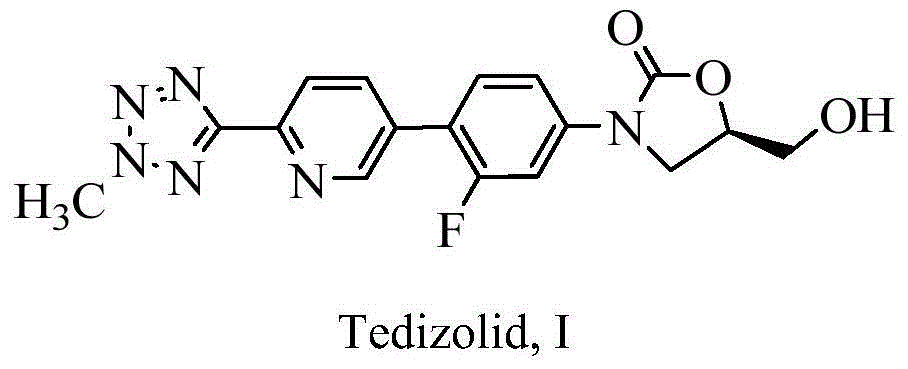
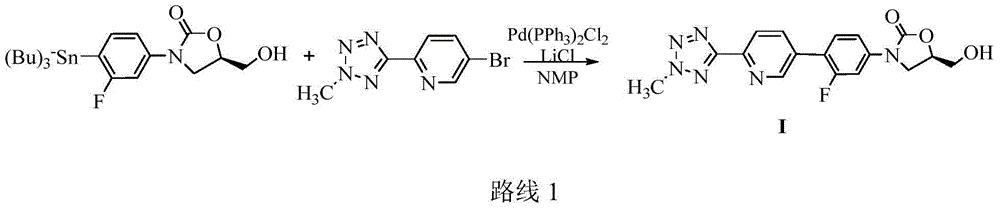
Process for preparing tedizolid
A technology of tedizolid and tetrazole, which is applied in the field of chemical drug synthesis, can solve the problems of high reagent cost, harsh reaction conditions, and high price, and achieve the effects of cheap and easy-to-obtain reagents, mild reaction conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1. Preparation of tedizolid
[0033]
[0034] Add 2-(2-methyl-2H-tetrazol-5-yl)-5-(4,4,5,5-tetramethyl-1,3-dioxaborane- 2-yl)pyridine (1.8g, 0.00627mol), 1,4-dioxane:water=5:1 mixed solvent 32mL, stirred, added (5R)-3-(4-bromo-3-fluorophenyl )-5-Hydroxymethyloxazolidin-2-one (1.82g, 0.00627mol), K 2 CO 3 (2.60g, 0.01881mol), degassed, nitrogen protection, adding Pd(PPh 3 ) 4 (0.72g, 0.000627mol), degas three times, nitrogen protection. Heat to 80°C, react for 20h, stop the reaction, add methanol:water=4:1 mixed solvent 20mL, stir for 1h, filter, filter cake with H 2 O was washed and dried to obtain 2.48 g of blue-gray solid, which was slurried with 30 mL of ethyl acetate for 5 h, filtered and dried to obtain 2.11 g of tedizolid with a yield of 90.95%.
[0035] 1 H NMR (DMSO-d 6 )δ8.93(s,1H), 8.23~8.18(m,2H), 7.76~7.68(m,2H), 7.53~7.51(dd,1H, J=1.2,8.4Hz), 5.26~5.23(t, 1H, J=5.6Hz), 4.78~4.74(m,1H), 4.47(s,3H), 4.18~4.13(m,1H), 3.92~3.88(m,1H), 3.7...
Embodiment 2
[0036] Example 2. Preparation of tedizolid
[0037] Add 2-(2-methyl-2H-tetrazol-5-yl)-5-(4,4,5,5-tetramethyl-1,3-dioxaborane- 2-yl)pyridine (1g, 0.00348mol), 1,4-dioxane:water=5:1 mixed solvent 18mL, stirred, added (5R)-3-(4-bromo-3-fluorophenyl) -5-Hydroxymethyloxazolidin-2-one (1.01g, 0.00522mol), K 2 CO 3 (1.44g, 0.0104mol), degassed, nitrogen protection, added PdCl 2 (dppf)DCM (0.28g, 0.000348mol), degassed 3 times, nitrogen protection. Heat to 80°C, react for 20h, stop the reaction, add 20mL H 2O, stirred for 1 h, filtered, the filter cake was washed with water, slurried with 20 mL of methanol: water = 4:1 mixed solvent for 3 h, filtered, and dried to obtain 1.15 g of tedizolid, with a yield of 89.15%.
Embodiment 3
[0038] Example 3. Preparation of tedizolid
[0039] Add 2-(2-methyl-2H-tetrazol-5-yl)-5-(4,4,5,5-tetramethyl-1,3-dioxaborane- 2-yl)pyridine (1g, 0.00348mol), DMF18mL, stirred, added (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one (1.01g ,0.00348mol), K 2 CO 3 (1.44g, 0.0104mol), degassed, nitrogen protection, added PdCl 2 (dppf)DCM (0.28g, 0.000348mol), degassed 3 times, nitrogen protection. Heat to 100°C, react overnight, stop the reaction, filter, add 50mL H to the filtrate 2 O, stir for 1h, filter, filter cake with H 2 O was washed, and 20 mL of mixed solvent of methanol:water=4:1 was used for beating for 3 hours, filtered, and dried to obtain 1.15 g of tedizolid, with a yield of 89.15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com