Tedizolid crystal and preparation method thereof
A technology of tedizolid and crystals, which is applied in the field of medicinal chemistry, can solve the problems of unfavorable industrial production of tedizolid phosphate, increase the preparation cost of tedizolid phosphate, and cumbersome preparation process, etc., and achieve easy large-scale production , stable quality and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] At room temperature, add 1.0 g of compound of formula I, 1.2 g of compound of formula II, 0.8 g of potassium carbonate, 60 mg of tricyclohexylphosphine and 72 mg of tris(dibenzylideneacetone) dipalladium (Pd 2 (dba) 3 ) catalyst, then add 4mL water and 15mL 1,4-dioxane, heat to reflux after vacuum and nitrogen replacement, keep warm and carry out SUZUKI coupling reaction under reflux state; when SUZUKI coupling reaction is complete (about 4 hours) , to end the reaction, filter the reaction solution while hot with diatomaceous earth, and naturally cool down and crystallize the collected filtrate to obtain 1.0g tedizolid crystals (off-white solid), the HPLC purity is 99.5%, and the molar yield is 78%.
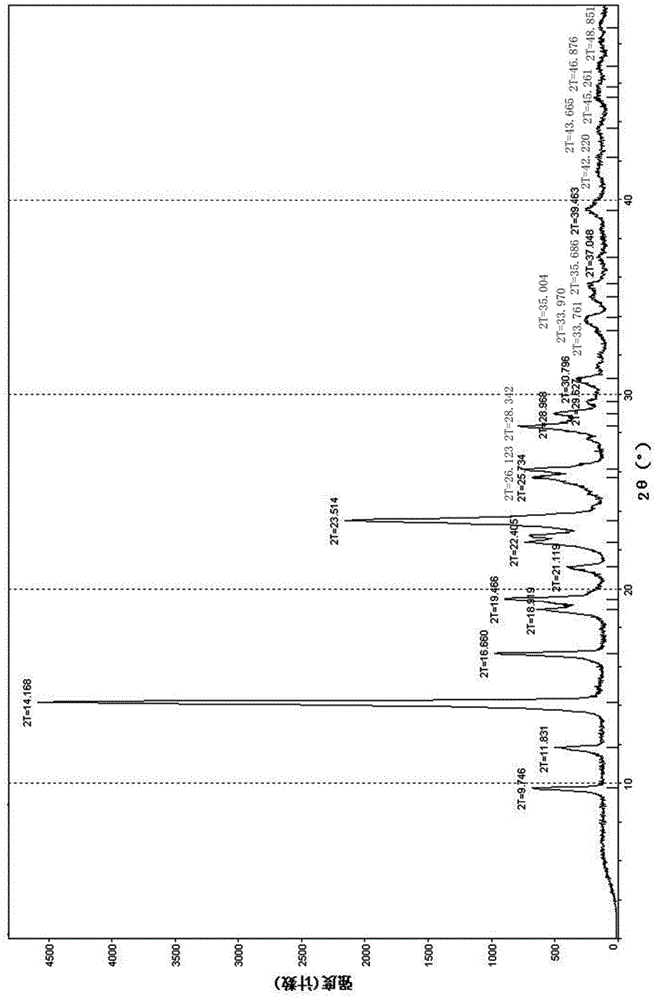
[0042] figure 1For the XRD spectrogram of the obtained tedizolid crystals, by figure 1 It can be seen that under X-ray powder diffraction, the crystal has a main characteristic peak with a peak intensity of 100% at a diffraction angle 2θ of 14.1±0.2°, and at a diffractio...
Embodiment 2
[0046] At room temperature, add 1.0 g of compound of formula I, 1.5 g of compound of formula II, 1.0 g of sodium carbonate, 60 mg of triphenylphosphine and 60 mg of Pd(PPh 3 ) 4 Catalyst, then add 10mL water and 5mL tetrahydrofuran, heat to reflux after vacuum and nitrogen replacement, keep warm and carry out SUZUKI coupling reaction under reflux state; when the SUZUKI coupling reaction is complete (about 8 hours), end the reaction, use diatomaceous earth The reaction solution was filtered while it was hot, and the collected filtrate was naturally cooled and crystallized to obtain 0.9 g of tedizolid crystals (off-white solid), with an HPLC purity of 99.1% and a molar yield of 70%.
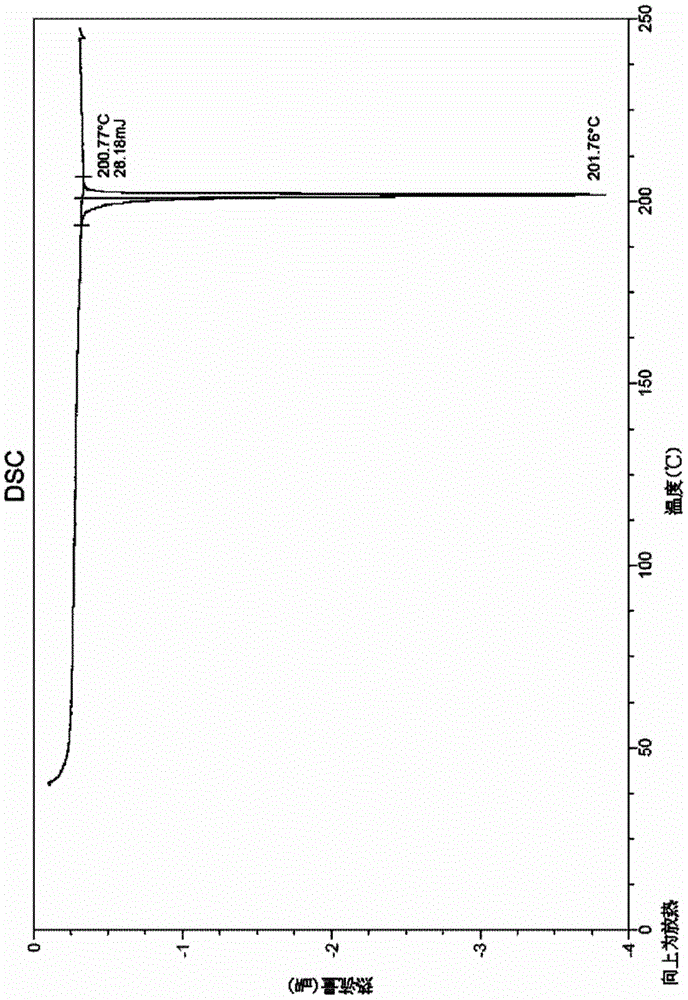
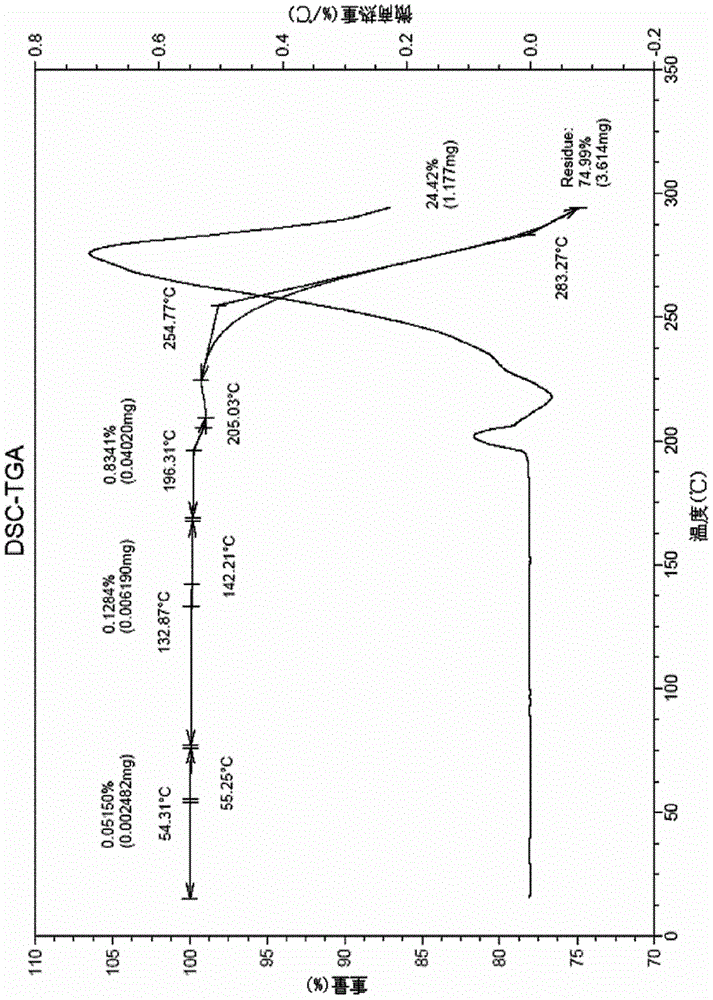
[0047] The tedizolid crystals obtained in this example also have figure 1 The XRD spectrum features shown and figure 2 The characteristics of the DSC spectrum shown and image 3 Characteristic TGA spectra shown.
Embodiment 3
[0049] At room temperature, add 1.0 g of compound of formula I, 1.1 g of compound of formula II, 1.0 g of triethylamine, 30 mg of triphenylphosphine and 30 mg of PdCl into the vessel 2 (PPh 3 ) 2 Catalyst, then add 2mL water and 20mL toluene, heat to reflux after vacuum and nitrogen replacement, keep warm and carry out SUZUKI coupling reaction under reflux state; when SUZUKI coupling reaction is complete (about 8 hours), end the reaction, use diatomaceous earth The reaction solution was filtered while it was hot, and the collected filtrate was naturally cooled and crystallized to obtain 1.0 g of tedizolid crystals (off-white solid), with an HPLC purity of 99.3% and a molar yield of 78%.
[0050] The tedizolid crystals obtained in this example also have figure 1 The XRD spectrum features shown and figure 2 The characteristics of the DSC spectrum shown and image 3 Characteristic TGA spectra shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com