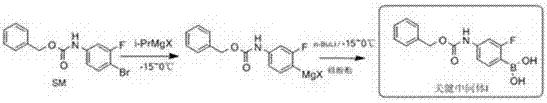
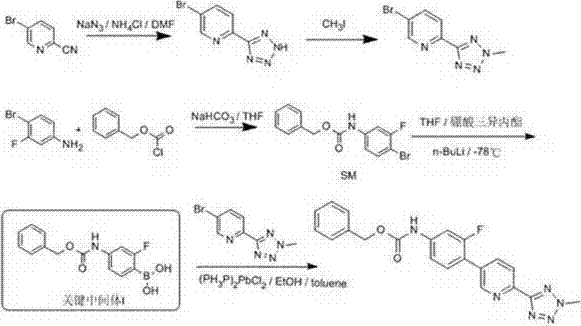
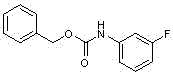
Novel synthesis method for key intermediate of tedizolid
A kind of technology of tedizolid and intermediate, which is applied in the field of raw medicine preparation, and achieves the effects of reducing the production of debrominated impurities, high yield and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Dissolve the starting material SM (50g, 0.155mol) in 500ml of anhydrous tetrahydrofuran, cool the reaction solution to -10°C, add isopropylmagnesium bromide (0.156mol) in tetrahydrofuran solution dropwise, and stir the reaction at -10°C 3h, add n-butyl lithium n-hexane solution (0.158mol) dropwise, stir at -10°C for 1h, add trimethyl borate (19.3g, 0.186mol) dropwise, stir at -10°C for 2h, thin-layer chromatography After detecting the reaction of the raw materials, add 250ml of water dropwise, stir and separate the layers, extract the water layer with ethyl acetate until there is no product in the water layer, combine the organic layers, wash with saturated brine, dry and concentrate to dryness to obtain 40.3g of white powdery intermediate I , the yield is 90%, and the purity is 99%.
Embodiment 2
[0038] The starting material SM (200g, 0.62mol) was dissolved in 2000ml of anhydrous tetrahydrofuran, the reaction solution was cooled to -10°C, a solution of isopropylmagnesium chloride (1.24mol) in tetrahydrofuran was added dropwise, and the reaction was stirred at -10°C for 5h. Add n-butyllithium n-hexane solution (0.93mol) dropwise, stir and react at -10°C for 1h, add trimethyl borate (1.24mol) dropwise, stir and react at -10°C for 2h, the reaction of raw materials is detected by thin layer chromatography, Add 1000ml of water dropwise, stir and separate the layers, extract the aqueous layer with dichloromethane until there is no product in the aqueous layer, combine the organic layers, wash with saturated brine, dry and concentrate to dryness to obtain 170.2g of white powdery intermediate I, yield 95% , with a purity of 98.9%.
Embodiment 3-7
[0040] According to the general method described in Example 1, the yield and purity of the tedizolid key intermediate I obtained by changing the technical conditions of each reaction are summarized in the following table:
[0041] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com