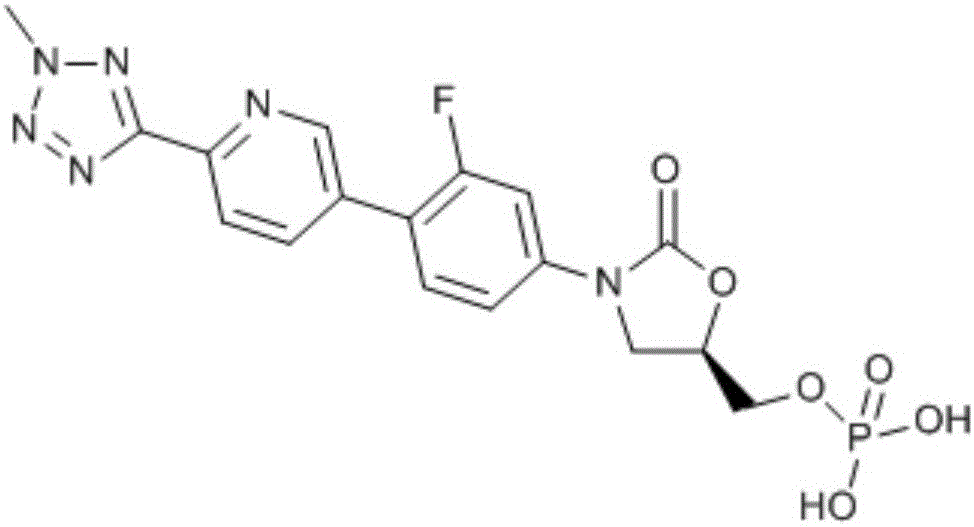
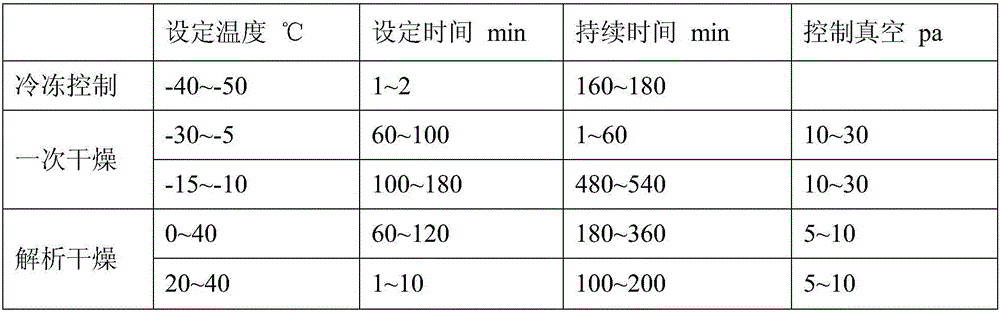
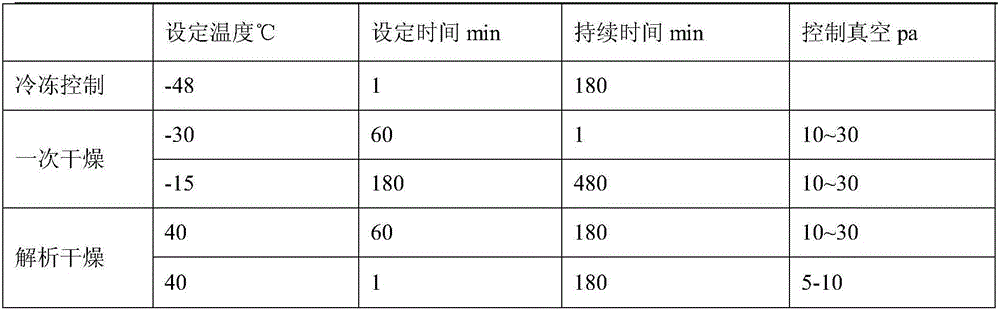
Tedizolid phosphate freeze-dried powder injection
A technology of tedizolid phosphate and freeze-dried powder injection, which is applied in the field of medicine, can solve problems such as the decrease of the clarity of the reconstitution solution, the impact on product quality, and the increase of related substances, so as to achieve high safety in clinical use, stable product quality, The effect of reducing the generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] prescription
[0041] tedizolid phosphate 200mg Mannitol 105mg sodium hydroxide solution 1mol / L Hydrochloric acid solution 1mol / L activated carbon 0.1% Water for Injection 2ml
[0042] craft
[0043] a) Inject 50% of the prescribed amount of water for injection into the liquid preparation tank, cool down to room temperature, add the prescribed amount of tedizolid phosphate to disperse for 5 minutes, add sodium hydroxide solution until the pH of the liquid is 7.50-7.70, and continue stirring for 30 minutes, Add mannitol to dissolve, and adjust the pH value to 7.70-7.80 with 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution.
[0044] b) Add 0.1% activated carbon to the medicinal solution in step a), stir and absorb for 20 minutes, and filter with 0.45 titanium rod.
[0045] c) After decarburization, water for injection is added to the medicinal solution to 100% of the prescription volume, mixed evenly, ...
Embodiment 2
[0053] prescription
[0054]
[0055] craft
[0056] a) Inject 50% of the prescribed amount of water for injection into the liquid preparation tank, cool down to room temperature, add the prescribed amount of tedizolid phosphate to disperse for 5 minutes, add 3mol / L sodium hydroxide solution until the pH of the liquid is 7.50-7.70, and continue stirring After 30 minutes, add mannitol to dissolve, and adjust the pH value to 7.70-7.80 with 3mol / L sodium hydroxide solution or 3mol / L hydrochloric acid solution.
[0057] b) Add 0.2% activated carbon to the medicinal solution in step a), stir and absorb for 20 minutes, and filter with 0.45 titanium rod.
[0058] c) After decarburization, water for injection is added to the medicinal solution to 100% of the prescription volume, mixed evenly, and the medicinal solution is filtered through a 0.22um+0.22um microporous membrane.
[0059] d) Intermediate test, after the test is qualified, it will be filled with 2ml / cartridge.
[006...
Embodiment 3
[0065] prescription
[0066]
[0067] craft
[0068] a) Inject 50% of the prescribed amount of water for injection into the liquid preparation tank, cool down to room temperature, add the prescribed amount of tedizolid phosphate to disperse for 5 minutes, add 5 mol / L sodium hydroxide solution until the pH of the liquid is 7.50-7.70, and continue stirring After 30 minutes, add mannitol to dissolve, and adjust the pH value to 7.70-7.80 with 5mol / L sodium hydroxide solution or 5mol / L hydrochloric acid solution.
[0069] b) Add 0.5% activated carbon to the medicinal solution in step a), stir and absorb for 20 minutes, and filter with 0.45 titanium rod.
[0070] c) After decarburization, water for injection is added to the medicinal solution to 100% of the prescription amount, mixed evenly, and the medicinal solution is filtered twice through a 0.22um microporous membrane.
[0071] d) Intermediate test, after the test is qualified, it will be filled with 2ml / cartridge.
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com