Preparation method for tedizolid
A technology of tedizolid and tedizolid phosphate, which is applied in the field of medicinal chemistry synthesis, can solve the problem of not finding tedizolid and the like, and achieves the effects of mild conditions, mild reaction conditions and strong reaction selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Formula II: Preparation of 5-bromo-2-(2H-tetrazol-5-yl)pyridine
[0039] Add 5-bromo-2-cyanopyridine (50 g, 273.22 mmol, 1 weight), N, N-dimethylformamide (400 ml, 8 volumes), chlorine Ammonium chloride (21.52g, 409.83mmol, 1.5 equivalents), sodium azide (26.64g, 409.83mmol, 1.5 equivalents), the oil bath temperature was set at 75°C (target temperature 80°C), the temperature reached 80°C within 20 minutes, and the reaction Continue self-heating to 84°C. After 1 hour, HPLC analysis shows that the initial raw materials are completely consumed, and the ammonium tetrazolium salt content is 92.1%, which means that the reaction is complete. The mixture was cooled, vacuum filtered at room temperature, the filter cake was washed with isopropanol (50ml, 1 vol), and dried under vacuum at 55°C to obtain 52.3g of white solid, yield 81.97%, HPLC: 95.7%.
Embodiment 2
[0040] Example 2 Preparation of Formula II 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine
[0041]In a 1L three-neck flask equipped with mechanical stirring, add tetrazolium ammonium salt (52.3g, 222.56mmol, 1 weight), tetrahydrofuran (313ml, 6 volumes), N,N-dimethylformamide (105ml , 2 volumes) and sodium hydroxide powder (22.56 g, 556.4 mmol, 2.5 equiv). The maximum internal temperature of the reaction device was allowed to be 10°C, and then methyl iodide (78.9g, 556.4, 2.5 equivalents) was added dropwise, keeping the temperature below 10°C, and the addition of methyl iodide was completed within 40 minutes. After the dropwise addition was completed, remove the ice bath, install a thermocouple heater and a reflux cooler on the reaction device, set the external temperature to 30°C, and continue the reaction to self-heat to 44°C. After 4 hours, the reaction was detected by HPLC, ammonium tetrazolium The salt was completely consumed and the reaction was judged complete. The mix...
Embodiment 3
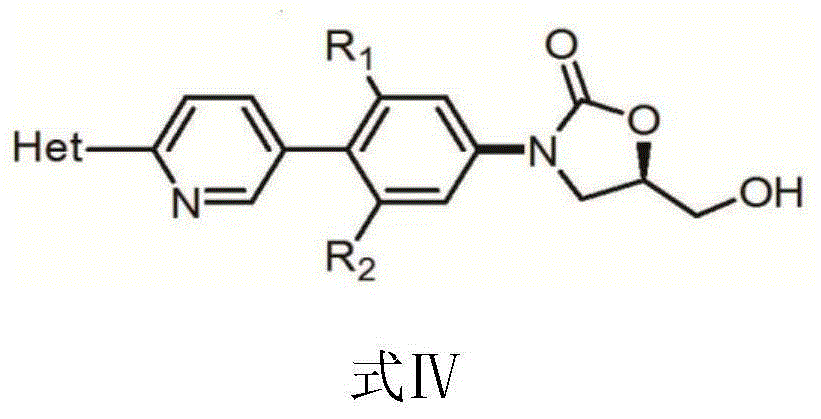
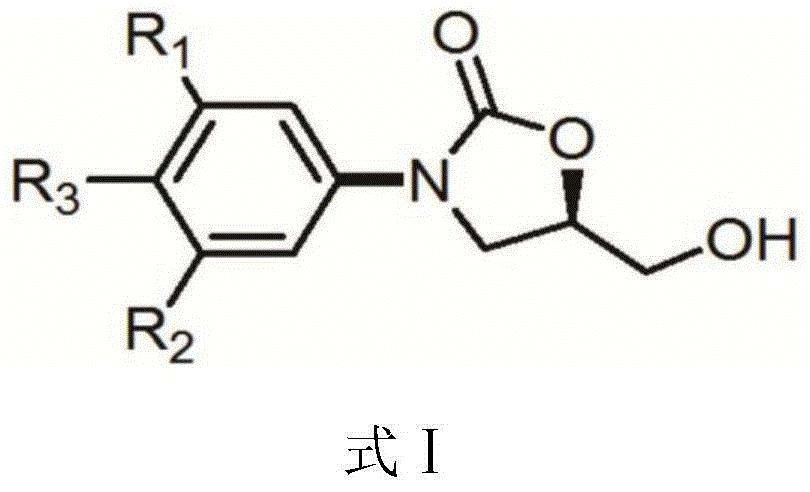
[0045] Example 3: (5R)-3-[3-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl Synthesis of Formula Ⅰ of ]-5-(Hydroxymethyl)-2-oxazolidinone
[0046] Add (5R)-3-(4-iodo-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one (10g, 29.67 mmol, 1 weight), double pinacol borate (32.90 mmol), potassium acetate (8.74 g, 89.01 mmol), dioxane (100 ml, 10 volumes).
[0047] Nitrogen gas bubbled for 20 minutes to replace the oxygen and water in the there-necked flask, and then added [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex (1.09g, 1.48mmol, 5%), continue nitrogen bubbling for 5 minutes, set the temperature to 80°C, raise the temperature to 80°C within 30 minutes, HPLC analysis after 3 hours, the initial raw materials have completely reacted, tedizolid intermediate I; (5R)-3 -[3-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-5-(hydroxymethyl) - The content of 2-oxazolidinone is 93.4%. It is judged that the reaction is complete. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com