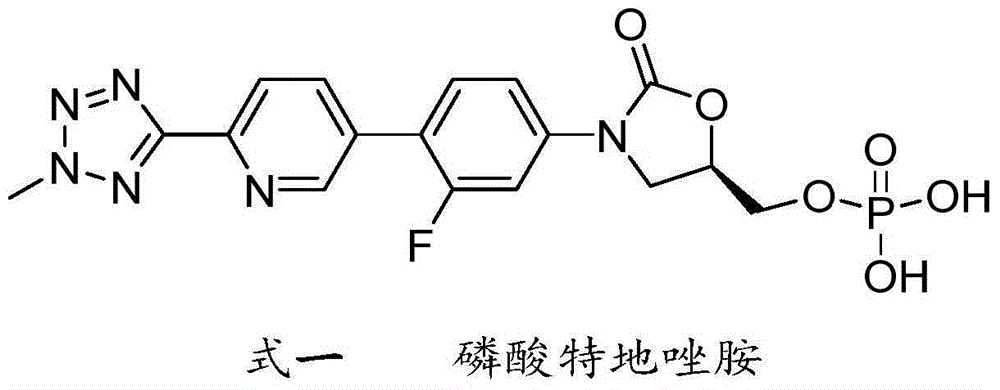
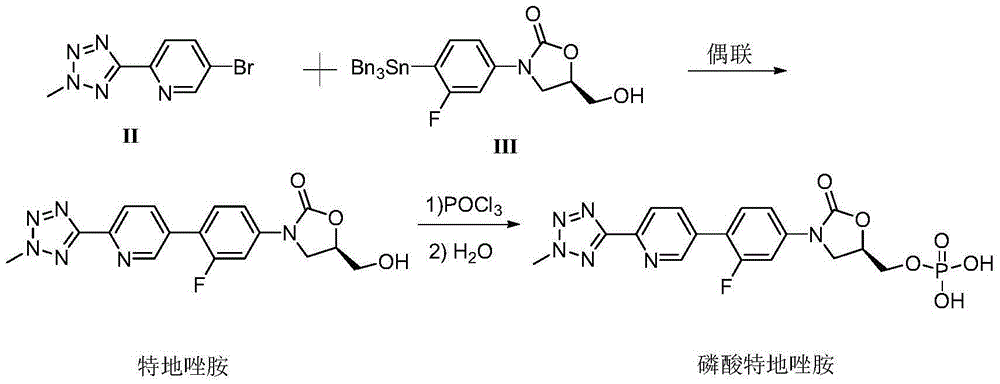
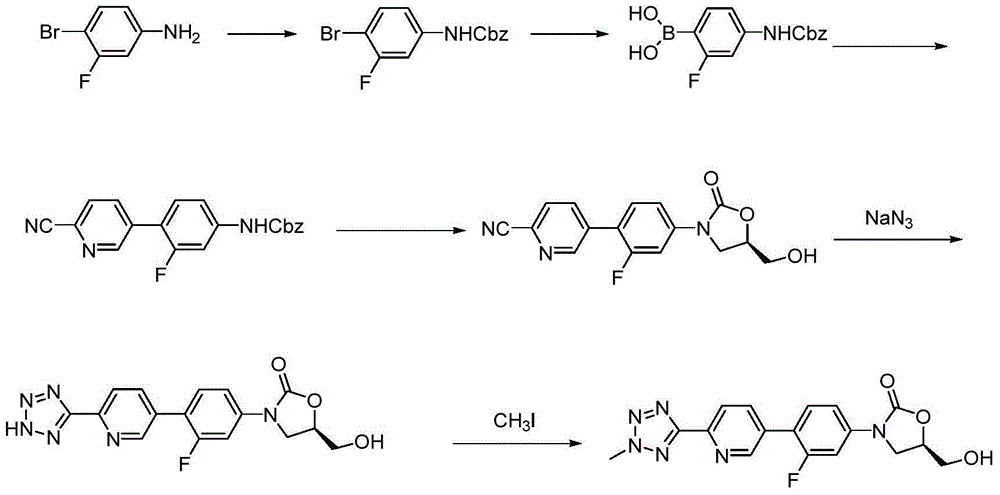
Method for compounding tedizolid phosphate
A technology of tedizolid phosphate and its synthesis method, which is applied in the field of pharmaceutical synthesis, can solve the problems of difficult access and high price of tedizolid, and achieve the effects of simple operation, environmental friendliness and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A method for synthesizing tedizolamide phosphate, comprising the following steps:
[0045] In the first step, the synthetic route of compound 4 is as follows:
[0046]
[0047] The specific method is: under the protection of nitrogen, add sodium hydrogen (NaH) (0.22g, 8.9mmol) and 40mL of dry tetrahydrofuran to a three-necked reaction flask, control the temperature at about 0°C, add compound 5 (2.50g, 7.4mmol), After stirring for 10 min, add dropwise 15mL solution of diethyl chlorophosphate (compound 6) (1.53g, 8.9mmol) in dry tetrahydrofuran. After the dripping is completed, stir at room temperature for 4h. TLC monitors the completion of the reaction. Add 100mL ice water to the system. It was extracted twice with 100 mL of ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated to obtain 43.26 g of compound.
[0048] 1 H-NMR (400MHz, DMSO-d 6 ,δ / ppm): 7.81~7.77(t,1H), 7.58~7.54(dd,1H), 7.22~7.19(dd,1H), 4.83~4.72(m,1H), 4.25~4.1...
Embodiment 2
[0058] A method for synthesizing tedizolamide phosphate, comprising the following steps:
[0059] In the first step, the synthetic route of compound 4 is as follows:
[0060]
[0061] The specific method is: under the protection of nitrogen, add sodium hydrogen (0.22g, 8.9mmol) and dry tetrahydrofuran 40mL into a three-necked reaction flask, control the temperature at about 0℃, add compound 5 (2.14g, 7.4mmol), stir for 10min , Add dropwise a 30mL solution of dibenzyl chlorophosphate (compound 6) (2.63g, 8.9mmol) in dry tetrahydrofuran, after dropping, stir at room temperature for 4h, TLC monitors the completion of the reaction, and add 100mL of ice water to the system. 100 mL of ester was extracted twice, the organic phases were combined, dried with anhydrous sodium sulfate, and concentrated to obtain 43.73 g of compound.
[0062] 1 H-NMR(400MHz,DMSO-d 6 ,δ / ppm): 7.80~7.76(t,1H), 7.57~7.53(dd,1H), 7.34~7.28(m,10H), 7.21~7.18(dd,1H), 5.08~4.97(m,4H) , 4.84~4.73(m,1H), 4.26~4.14(m,2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com