Novel oxazolidinone compound and preparation method thereof
A compound and composition technology, applied in the field of new oxazolidinone compounds and their preparation, can solve the problems of linezolid with obvious side effects, increased risk of drug resistance, lack of platelets, etc., and achieve excellent antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
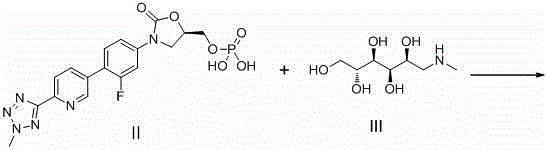
[0051] Example 1 : Preparation of tedizolid phosphate dimeglumine (formula I' compound)
[0052]
[0053]
[0054] Add 10g (0.0222mol) tedizolid phosphate to 100ml water, add 8.67g (0.0444mol) N-methyl-D-glucamine dropwise to the above solution, after the dropwise addition, stir at room temperature for 1~2h , filtered the reaction solution, collected the mother liquor, and freeze-dried the mother liquor to obtain 18.54 g (99.3% yield) of a white solid, namely tedizolid dimethylglumine phosphate.
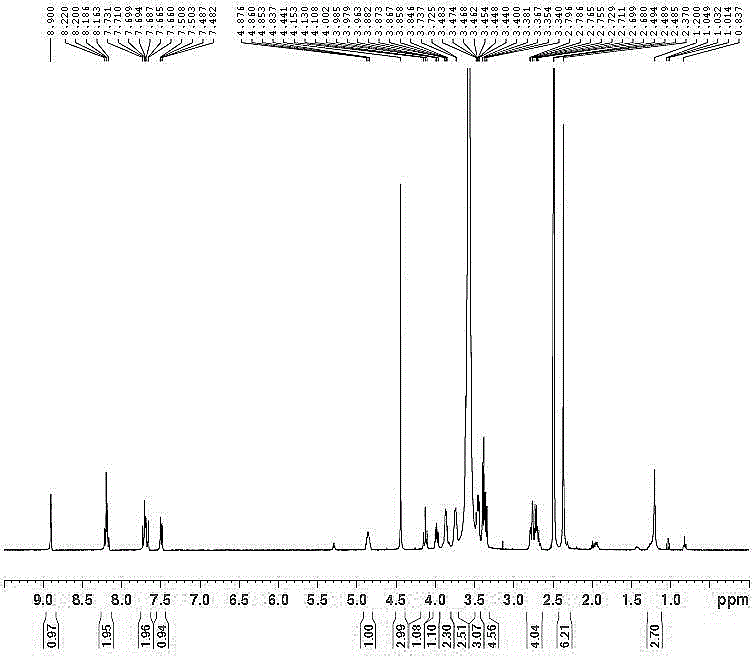
[0055] 1 H-NMR (DMSO-D 6 +D 2 O): δ8.90(s,1H), 8.22~8.16(m,2H), 7.73~7.66(m,2H), 7.51~7.48 (dd,1H), 4.88~4.84(m,1H), 4.44 ( s,3H), 4.15~4.10(t,1H), 4.00~3.96(dd,1H), 3.88~3.85(m,2H), 3.74~3.73(m,3H), 3.48~3.44(m,3H), 3.40~3.34(m,5H), 2.80~2.68(m,4H), 2.37~2.68(s,6H). Tedizolid Dimeglumine Phosphate 1 Please see Appendix 1 for the H-NMR spectrum.
Embodiment 2
[0056] Example 2 : Preparation of tedizolid phosphate dimeglumine (formula I' compound)
[0057] Add 10g (0.0222mol) tedizolid phosphate to 100ml water, add 13.0g (0.0666mol) N-methyl-D-glucamine dropwise to the above solution, after the dropwise addition, stir at room temperature for 1~2h , filtered the reaction solution, collected the mother liquor, and freeze-dried the mother liquor to obtain 18.57 g of white solid (yield 99.5%), namely tedizolid dimethylglumine phosphate.
[0058] 1 H-NMR (DMSO-D 6 +D 2 O): δ8.90(s,1H), 8.22~8.16(m,2H), 7.73~7.66(m,2H), 7.51~7.48 (dd,1H), 4.88~4.84(m,1H), 4.44 ( s,3H), 4.15~4.10(t,1H), 4.00~3.96(dd,1H), 3.88~3.85(m,2H), 3.74~3.73(m,3H), 3.48~3.44(m,3H), 3.40~3.34(m,5H), 2.80~2.68(m,4H), 2.37~2.68(s,6H).
Embodiment 3
[0059] Example 3 : Preparation of tedizolid monomeglumine phosphate (formula I'' compound)
[0060]
[0061] Add 10g (0.0222mol) tedizolid phosphate to 100ml water, add 4.34g (0.0222mol) N-methyl-D-glucamine dropwise to the above solution, after the dropwise addition, stir at room temperature for 1~2h , filtered the reaction solution, collected the mother liquor, and freeze-dried the mother liquor to obtain 14.2 g of white solid (yield 98.9%), namely tedizolid monomeglumine phosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com