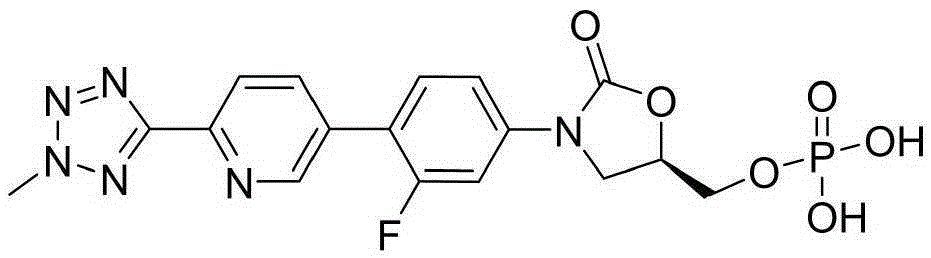
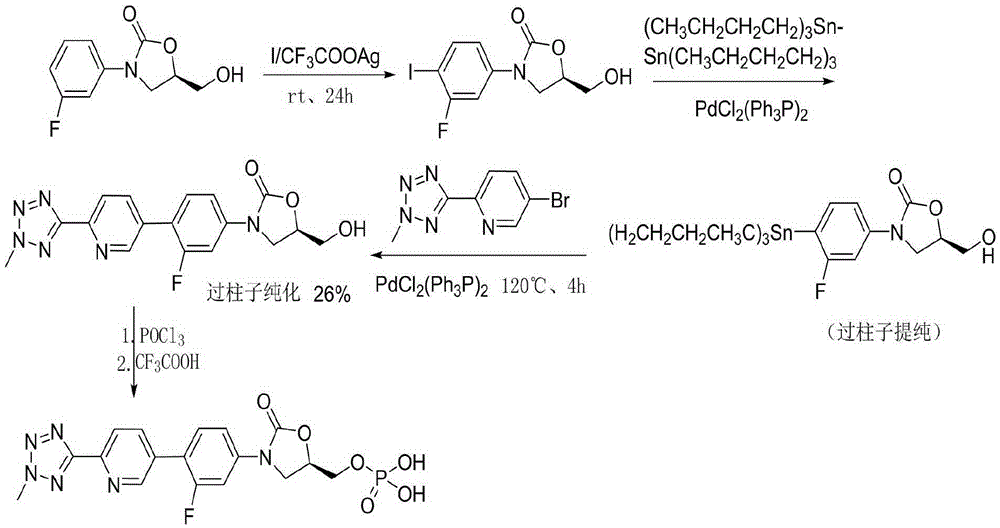
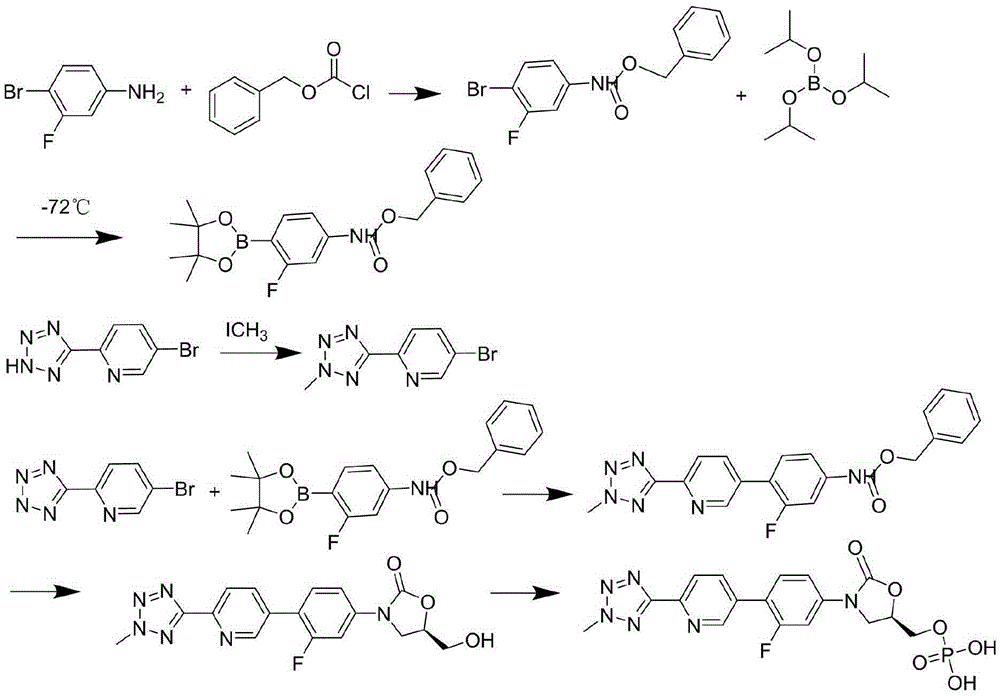
Preparation method of tedizolid phosphate
A technology for tedizolid phosphate and a compound, which is applied in the field of chemical drug synthesis, can solve problems such as being unsuitable for industrial production, harsh synthesis conditions, harsh reaction conditions, etc., and achieves the effects of avoiding alkylating reagents, short synthesis steps, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Compound 3-[3-fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridyl]phenyl]-5-(hydroxymethyl Synthesis of base)-2-oxazolidinone
[0033] In a 3000mL four-neck round-bottomed flask, install mechanical stirring, reflux condenser, thermometer, and add 1500mL tetrahydrofuran and 100g (5R)-3-(4-bromo-3-fluorophenyl) to the round-bottomed flask under nitrogen protection. -5-hydroxymethyloxazolidin-2-one, 105g2-(2-methyl-2H-tetrazol-5-yl)pyridine-5-boronic acid pinacol ester, 28g tetrakis(triphenylphosphine)palladium , start stirring until the solid dissolves, add 140 g of prepared potassium carbonate solution dissolved in a small amount of water, heat up to reflux, stir and react for 12 hours, and cool down to room temperature. Suction filtration, the filter cake was washed with water and then washed with acetone. The filter cake was vacuum dried at 45°C for 48 hours to obtain 80.2 g of product. Yield: 62.8%.
[0034] (2) Synthesis of compound tedizolid phosphate crude pr...
Embodiment 2
[0048] (1) Compound 3-[3-fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridyl]phenyl]-5-(hydroxymethyl Synthesis of base)-2-oxazolidinone
[0049] In a 3000mL four-neck round bottom flask, install mechanical stirring, reflux condenser, thermometer, and add 1500mL toluene and 100g (5R)-3-(4-bromo-3-fluorophenyl) to the round-bottom flask under argon protection )-5-hydroxymethyloxazolidin-2-one, 105g2-(2-methyl-2H-tetrazol-5-yl)pyridine-5-boronic acid pinacol ester, 28g dichlorobis(triphenylphosphine ) palladium, start stirring until the solid dissolves, add a small amount of water-soluble potassium carbonate solution prepared in 140g, heat up to reflux, stir and react for 12 hours, and cool to room temperature. After suction filtration, the filter cake was washed with water and then washed with a mixture of methanol and ethanol. The filter cake was vacuum dried at 45°C for 48 hours to obtain 81.3 g of product. Yield: 63.7%.
[0050] (2) Synthesis of compound tedizolid phosphat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com