Key intermediate for preparing tedizolid phosphate, and preparation method of key intermediate
A technology of tedizolid phosphate and intermediates, applied in the field of drug synthesis, can solve the problems of potential safety hazards, impact on the quality of finished raw materials, high corrosiveness of phosphorus oxychloride, etc., and achieves simple operation, mild reaction conditions, and easy purification. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of formula I key intermediate (R=phenyl)
[0040] Add 1.1g of sodium hydride and 50mL of dry tetrahydrofuran into a 100mL round-bottomed flask in sequence, and drop 5g of the dry tetrahydrofuran solution of the compound of formula II into the reaction flask under the conditions of ice bath and nitrogen protection. The reaction was stirred at room temperature for 5 hours; subsequently, 11 g of tetrabenzyl pyrophosphate was added to the reaction liquid in batches, and the reaction was stirred at room temperature for 10 hours.
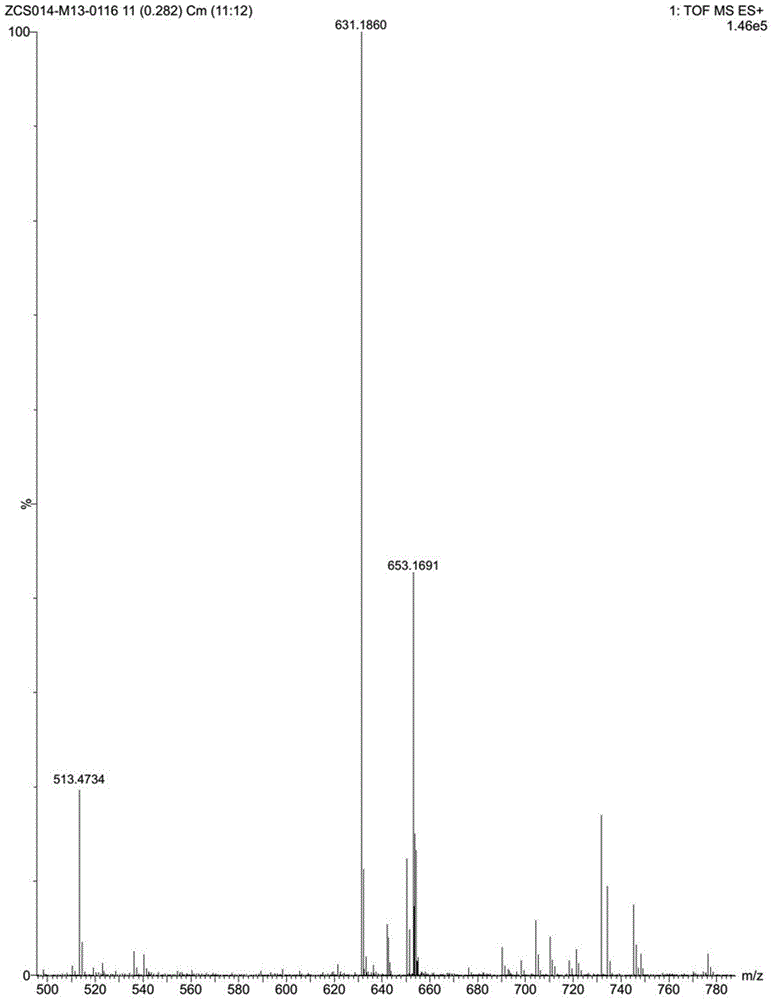
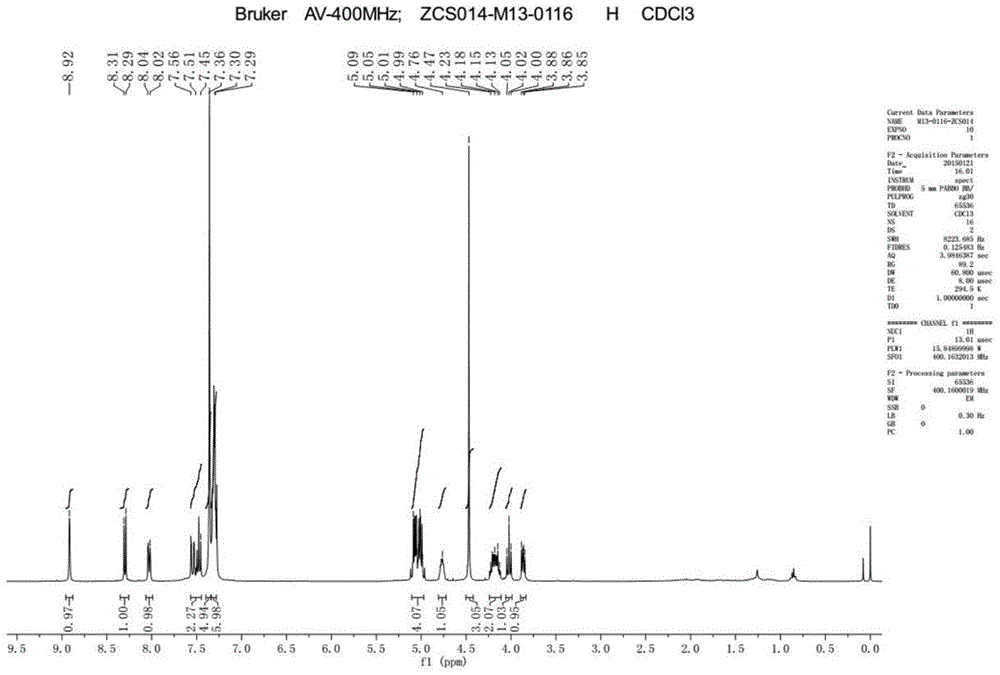
[0041] TLC detects that the reaction is complete. Slowly add ice water to the reaction solution to quench the reaction, extract with ethyl acetate, separate layers, dry and concentrate the organic layer, and recrystallize the residue with ethyl acetate / petroleum ether to obtain about 7 g of a light yellow solid, yield: 82 %. 1 H-NMR (CDCl 3 ,400MHz): δ=8.92(s,1H),8.30(d,J=8.0Hz,1H),8.04~8.02(m,1H),7.55~7.45(m,2H),7.36~7.2...
Embodiment 2
[0042] Example 2 Preparation of tedizolid phosphate
[0043] 3g of the key intermediate of formula I (R=phenyl), 0.3g of Pd / C, and 30mL of methanol were sequentially added into a 50mL round-bottomed flask, and stirred for about 6 hours under hydrogen gas at room temperature. TLC monitored the completion of the reaction. Add sodium hydroxide solution to the reaction to adjust the pH of the reaction solution to 9-10, filter, wash the filter cake with water, and collect the filtrate.
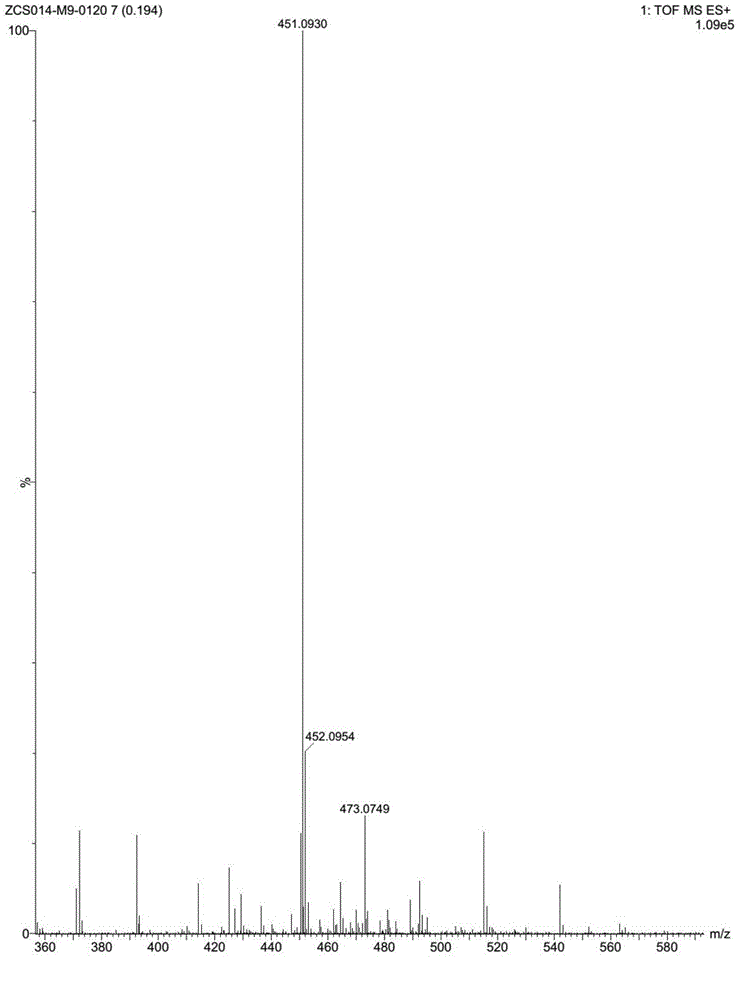
[0044] Under stirring conditions, the pH of the solution was adjusted to 2-3 with hydrochloric acid, and a solid was precipitated. Filtrate, wash with water, collect the solid, and recrystallize to obtain about 1.5 g of a white solid, yield: 71%. 1 H-NMR (d 6 -DMSO, 400MHz): δ=8.94(s,1H), 8.24~8.18(m,2H), 7.78~7.68(m,2H), 7.53~7.50(m,1H), 4.96~4.95(m,1H) ,4.48(s,3H),4.23(t,J=8.0Hz,1H),4.14~4.02(m,2H),3.94~3.90(m,1H)ppm; HRMS-ESI(m / z):451.0930( M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com