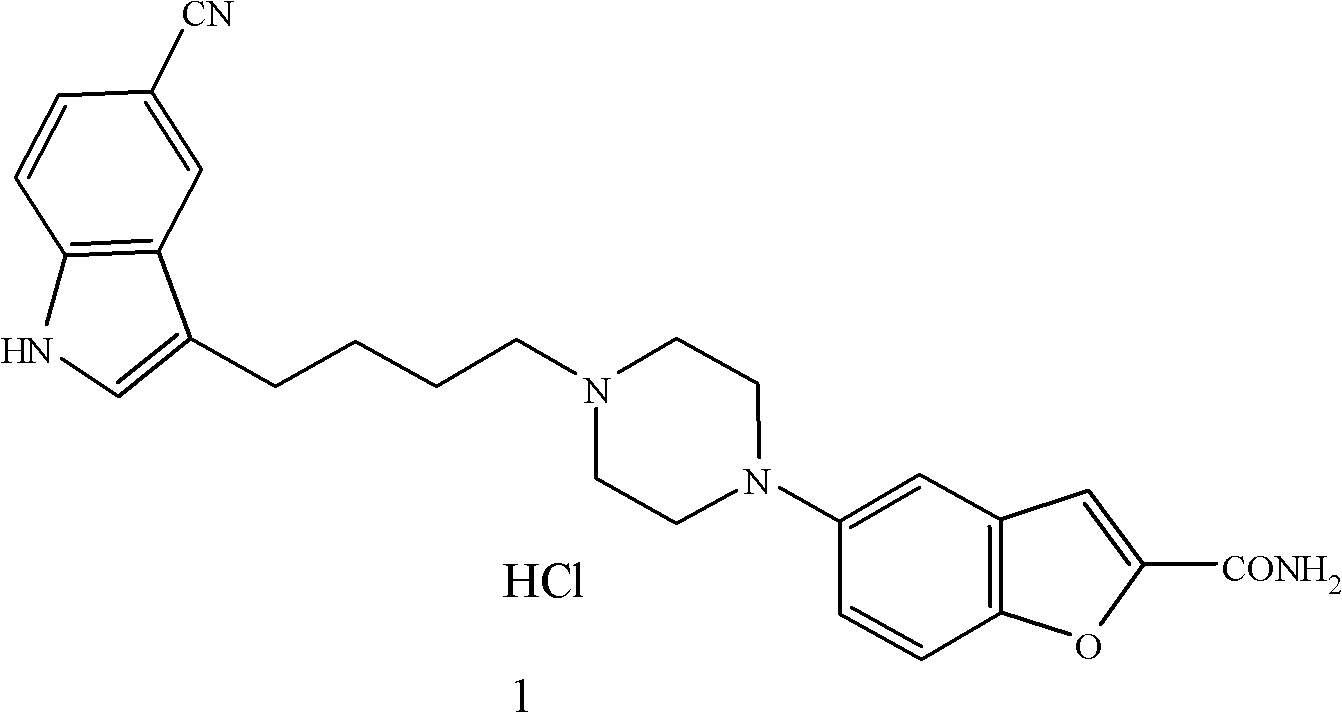
Method for preparing anti-depression medicine vilazodone
A vilazodone, antidepressant technology, applied in the direction of organic chemistry and the like, can solve the problems of unsuitable industrial production, low synthesis yield, high price, etc., and achieves great application value, high reaction yield, and easily available raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
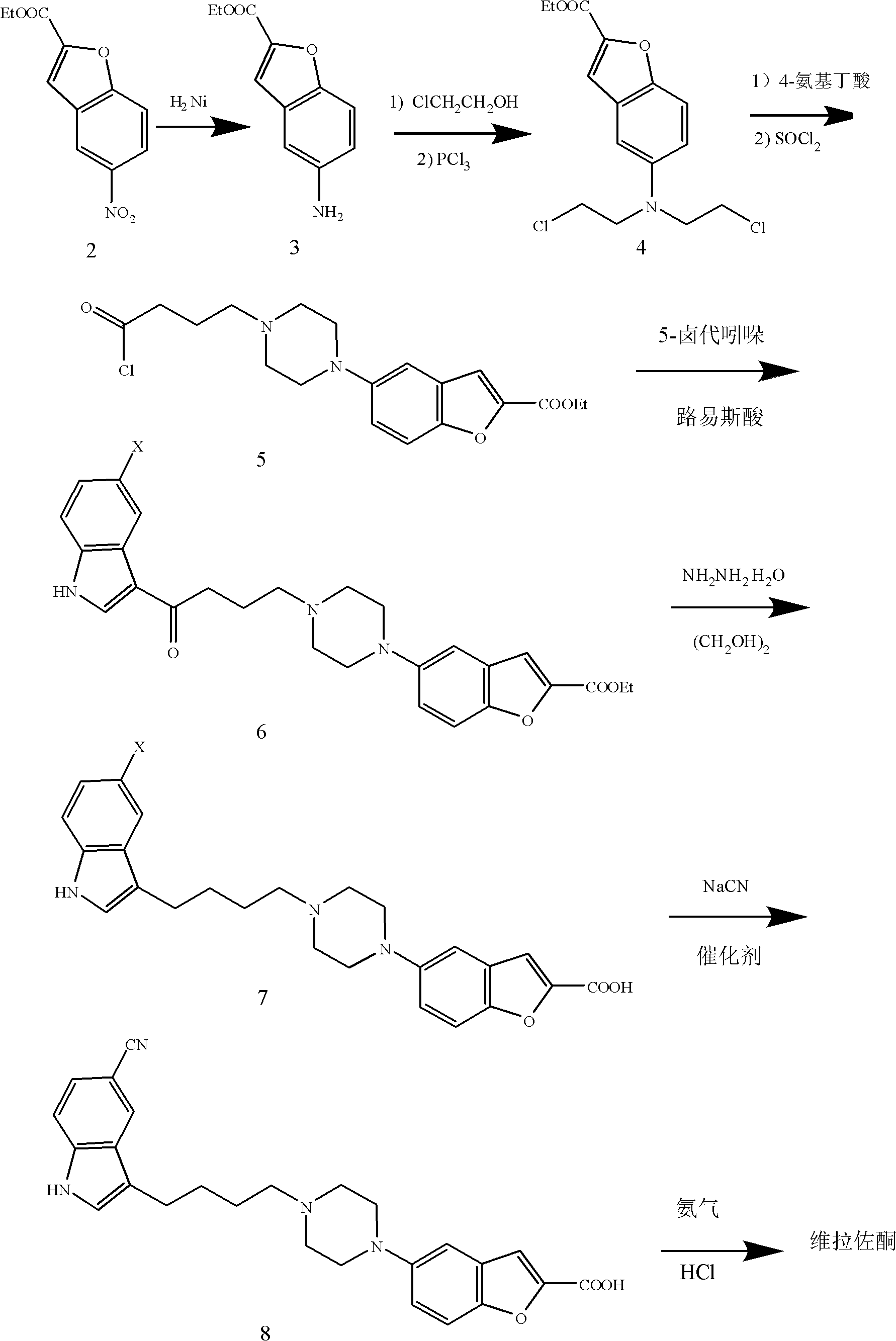
[0032] (1) Preparation of ethyl 5-aminobenzofuran-2-carboxylate [formula 3 compound]
[0033] 468g (2.0mol) of compound (2), 160g of Raney nickel and 10L of anhydrous methanol were added into a 30L small autoclave, and reacted for 12 hours under the pressure of 5atm and 20°C. The reaction solution was filtered to remove Raney nickel, and methanol was distilled off under reduced pressure. 392 g of crude product of the compound of formula 3 are obtained. The crude product was recrystallized from ethanol to obtain 380 g of the refined product compound of formula 3 as tan crystals. Yield: 93%. Melting point: 61°C.
[0034] (2) Preparation of 5-(bis(2-chloroethyl)amino)benzofuran-2-carboxylic acid ethyl ester [formula 4 compound]
[0035] Mix 40.8g (0.2mol) of the compound of formula 3, 50.5g (0.25mol) of triethylamine and 250ml of DMF, slowly add 38.64g (0.48mol) of 2-chloroethanol dropwise at 20°C, and heat to 90 °C, react for 3 hours. After cooling, the DMF solvent was dis...
Embodiment 2
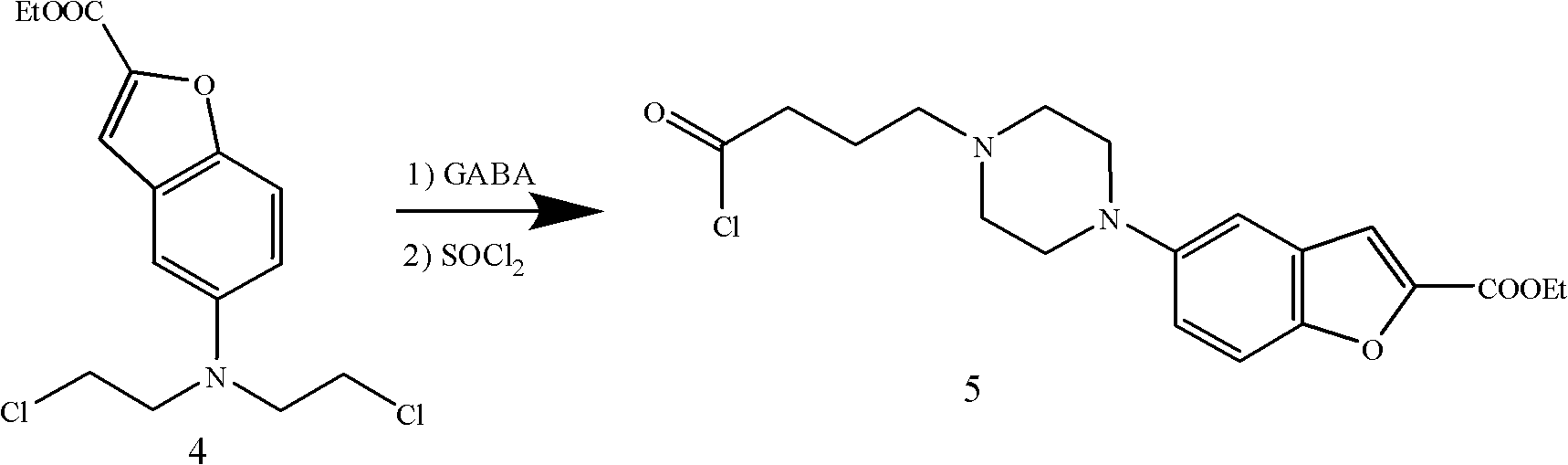
[0052] Except changing the reaction condition of formula 5 compound, other steps are identical with embodiment 1
[0053]
[0054] Mix 15.83g (0.05mol) of the compound of formula 4, 17.25g (0.125mol) of anhydrous potassium carbonate, and 100ml of DMF, and slowly add a mixed solution of 7.73g (0.075mol) of 4-aminobutyric acid and 25ml of DMF dropwise at 80°C. After the dropwise addition, the reaction was continued for 20 hours to the end of the reaction. The solvent was distilled off under reduced pressure, and 200 ml of dichloroethane was added, followed by washing with 50 ml x 3 times of water and 100 ml x 2 times of saturated brine. The organic layer was dried with anhydrous magnesium sulfate overnight, and the anhydrous magnesium sulfate was removed by suction filtration. 10 ml of thionyl chloride was added dropwise to the dried reaction liquid, and heated to reflux for 2 hours. After the reaction was completed, the remaining thionyl chloride and the solvent were disti...
Embodiment 3
[0056] Except changing the reaction condition of formula 6 compound, other steps are identical with embodiment 1
[0057] First put 4.4g (0.032mol) of anhydrous aluminum trichloride into 200ml of dichloroethane, cool down to 0°C, slowly add 11g (0.029mol) of the compound of formula 5 in 50ml of dichloroethane solution, and control the temperature at 10°C the following. After the dropwise addition was completed, the stirring was continued for 30 minutes, and then a solution of 6.27g (0.032mol) of 5-bromoindole in 30ml of dichloroethane was slowly added. The temperature is controlled between 0-5°C. React for 1.5 hours. The reaction solution was poured into 1L of ice water, and the base layer was separated, dried with anhydrous magnesium sulfate overnight, and the solvent was distilled off under reduced pressure to obtain a brown thick oil, which was recrystallized with isopropanol. 11.49 g of the compound of formula 6 was obtained, yield: 73.9%.
[0058] 1 H-NMR (d 6 -DMSO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com